The Ministry of Food and Drug Safety (MFDS) has warned consumers to be cautious about directly purchasing some foreign health supplements through online shopping malls at home and abroad.
The ministry inspected 30 products for the spring season targeting the improvement of fine dust, respiratory, and allergic diseases, sold directly overseas in online shopping malls in Korea and abroad. It identified 11 products with ingredients restricted from importation into Korea.
The inspections included ingredients claiming to improve and treat respiratory diseases (11 types, including azelastine and dexamethasone) and antihistamines for allergic diseases (35 types, including acrivastine and cyclizine).
As a result of the inspection, 11 products that claim to improve the efficacy and effectiveness of respiratory and allergic diseases were found to contain ingredients subject to an import ban. Two of them contained azelastine, dexamethasone, and chlorpheniramine, which are specialized pharmaceutical ingredients.
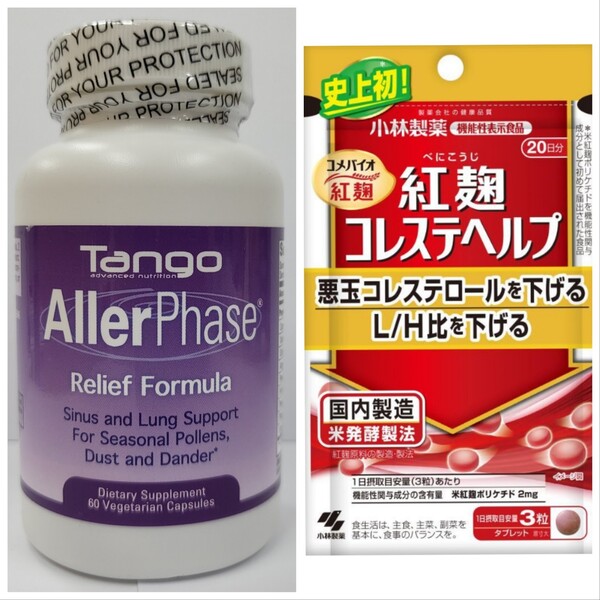
The azelastine-containing product is “Aller Phase Relief Formula,” a dietary supplement manufactured and distributed by Tango Advanced Nutrition in the U.S., and the dexamethasone- and chlorpheniramine-containing product is Margaritae Cough, manufactured and distributed by Shanghai Pharmaceutical Factory in China.
Azelastine, dexamethasone, and chlorpheniramine are pharmaceutical ingredients that relieve and treat allergy symptoms. If misused or abused, they can cause serious side effects, including drowsiness, cardiovascular system, and digestive system.
In addition, nine products were found to contain ingredients banned from being imported into Korea, including N-Acetyl Cystein, Andrographis paniculata, Machilus thunbergii, echinacea, heterostyly, Anemarrhena asphodeloide, goldenseal root, and vervine. The ministry said these ingredients can cause side effects, such as nausea, vomiting, and diarrhea, if misused or abused, so caution should be exercised.
Since 2008, the MFDS has designated ingredients and components of overseas direct-delivered foods (including narcotics, medicinal and herbal ingredients) that may pose a risk to public health as ingredients and components to be blocked from being brought into Korea to strengthen the safety management of these foods.
The ministry has taken measures to prevent the importation of 11 products containing ingredients subject to a domestic importation blockade. It has requested the Korea Customs Service (KCS) suspend customs clearance and the Korea Communications Standards Commission block access to online sales sites.
The regulator also called for consumers to pay attention to information that patients have developed kidney disease and other illnesses after consuming “red yeast rice” dietary supplements manufactured and sold by Japan's Kobayashi Pharmaceutical.
The five red yeast rice dietary supplement products were not officially imported into Korea. To prevent direct sales of these products overseas, MFDS asked domestic platforms to provide detailed information about them and refrain from selling them.
The 11 products containing ingredients and ingredients subject to import ban and the five red yeast rice dietary supplements announced by Kobayashi Pharma for recall can be found on the “Correct Direct Overseas Food Purchase” section of the ministry's "Food-Safe Country" website.
"Food bought directly from abroad cannot be guaranteed to be safe, and health functional foods that advertise specific 'efficacy and effectiveness' are likely to contain illegal drug ingredients, so special attention is required," the ministry said. "Consumers should check the “Correct Direct Overseas Food Purchase” website first to see if the product contains ingredients or ingredients that are subject to blocked importation into Korea, and do not purchase products registered as hazardous overseas direct purchase food."
The ministry said it would continue to expand inspections of items of concern and items of consumer interest and to provide consumers with information on precautions and risks when purchasing food directly from abroad.
In addition, in accordance with the 2024 Basic Plan for Manufacturing and Distribution Management of Drugs and Narcotics, the ministry will clarify the legal basis for regulating the importation of drugs by individuals from overseas and periodically review the items to be blocked at customs through the MFDS-KCS Information Sharing System.

