Pharmaceutical firm Handok allegedly encouraged pharmacists to carry out unlicensed medical practices during its promotional activities of Souvenaid, a new drink claiming to help treat dementia patients, a civic group said Thursday.
According to Barun Medicine Institute, comprising of some 20 young physicians, Handok recently sent out a promotion report called “Dementia and Pharmacy” to healthcare professionals.
“Pharmacies can play a role in managing dementia,” Handok’s release said. “Pharmacists are experts who understand dementia and who are capable of managing it. They can provide consultations for dementia and family support.”
The report also noted that pharmacies, where patients’ history of medications and risk factors are easily checked, could be utilized to spot symptoms of dementia at an early stage.
Criticizing the promotion report, Barun Medicine Institute pointed out that the Medical Service Act prohibits pharmacists’ medical inquiries and diagnosis. “Handok encouraged unlicensed medical practices for pharmacists to discover dementia early through questionnaires and consultations on patients and even to diagnose,” the institute said.
Article 27 of the Medical Service Act prohibits non-medical personnel from performing medical practices.
As pharmacists are not medical personnel, any activity by a pharmacist regarding the diagnosis of dementia is in breach of the Medical Service Act, the civic group said.
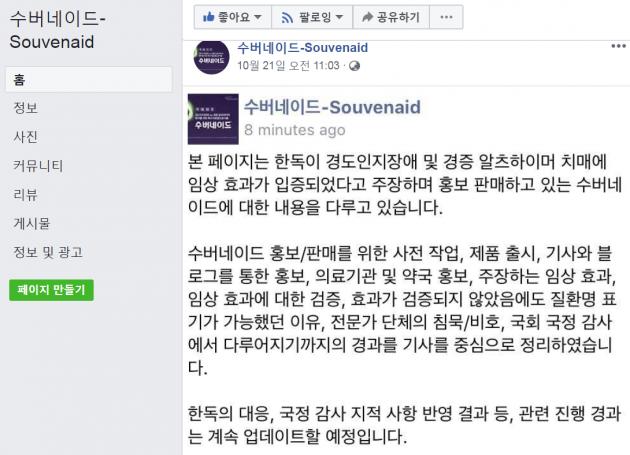
A Facebook page with a title “Souvenaid” appeared, seemingly aiming to monitor excessive marketing of the medical drink.
The page provides various data and internet links to pre-work for promotion and marketing of Souvenaid, Souvenaid-promoting news reports and blogs, advertisements at medical institutions and pharmacies, “clinical effectiveness” argued by Handok, verification of the efficacy, and reasons why the product labeled disease name despite questionable efficacy.
The page asserted that expert groups silently connived at Handok’s promotion of the drink and introduced news reports until the issue appeared during the National Assembly’s audit on the healthcare-related agencies.
The page said it would keep updating latest developments in Handok’s response and outcomes from the parliamentary audit.
The Facebook page is monitoring the product, not promoting it.
Recently, the Ministry of Food and Drug Safety raised the possibility that consumers might confuse Souvenaid as a pharmaceutical product.

