Wearable device ‘CART’ can detect 98% of unequal pulse, Sky Labs CEO says
Arrhythmia, caused by irregular pulses -- too fast (tachycardia), too slow (bradycardia) and unequal (atrial fibrillation) ones – are diseases that are especially difficult to diagnose. Doctors call it “silent assassin,” because the ailment accounts for 90 percent of sudden deaths and 20-30 percent of strokes.
The problem is people have to wear electrocardiogram (ECG) for a long time for correct diagnosis. And, even if patients feel the symptoms of arrhythmia and visit hospitals, medical workers sometimes fail to find them.

That also explains why experts think there are a considerable number of hidden patients of arrhythmia in Korea.
It is against this backdrop a local venture has made headlines by developing the technology to solve this problem. Doing that is “Sky Labs,” which has developed a wearable device to diagnose an arrhythmia.
Sky Labs CEO Lee Byung-hwan recently met with Korea Biomedical Review to explain how the wearable diagnosis equipment had come into being.
Lee said he felt the need for the device because none other than the CEO himself was suspected of having an arrhythmia. So he quit Samsung Electronics and decided to launch a business. Lee, who started Sky Labs in 2015, began to develop the device in earnest the following year.
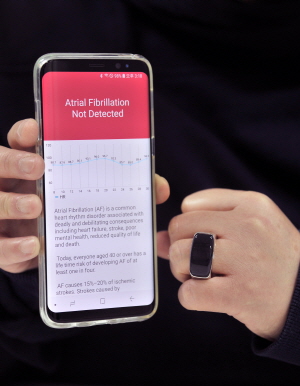
The device developed by Sky Labs is “CART (Cardio Tracker),” which looks like a ring. The company manufactured the existing ECG device in the form of a ring that can detect atrial fibrillation (AF).
While people are wearing the ring, they can continually confirm AF through an application, and also diagnose irregular heartbeats by themselves.
In clinical trials, CART recorded the 98 percent of accuracy. Given the accuracy of a wristband-type wearable device developed abroad stood at 95.3 percent, Sky Labs’ product has reliable accuracy, although it came to the market a little later.
To secure more evidence, however, the company is conducting trials, both at home and overseas. In Korea, Sky Labs, in cooperation with Seoul National University Hospital’s Circulatory Internal Medicine Department, is carrying out trials on 150 patients. Abroad, it is making similar tests in Charite University Hospital in Germany, the largest university hospital in Europe.
“Arrhythmia is a grave disease as the main cause of cardiac insufficiency, but its diagnosis rate is rather low, and the existing diagnosing methods had limitations by making it difficult for patients to lead normal lives,” Lee said. “Particularly, people have found it hard to diagnose arrhythmia because they don’t know when it will occur.”
Global pharmaceuticals are paying keen attention to the innovative device and its high diagnosis rate. Since Sky Labs was chosen as one of the final participants in “Grants4Apps Korea,” a program under the joint auspices of Bayer Korea and Korea Trade-Investment Promotion Agency (KOTRA), it has attracted massive investment from Bayer Global.
The company is also discussing business cooperation with another global drugmaker, Sanofi, with international insurance companies supporting it. Despite such an impressive network of collaborators, Sky Labs will take full charge of marketing.
“I am taking part in conferences of cardiology conferences to meet with medical professionals one by one,” Lee said. “I think the purchase rate of our product will go up if there are doctors’ prescriptions and directions. I learned that from collaboration with pharmaceuticals, found the way in it, and will be able to develop it further.”
Sky Labs’ next objective is to enter into global markets. Lee thinks that as foreign markets are larger and freer than the domestic market, the advance will help the company grow further.
In 2020 when the company launches its product, the market for diagnosing arrhythmia is expected to grow to 1 trillion won ($878 million), and Sky Labs plans to raise its market share to 25-40 percent.
Sky Labs’ profit model is subscription, in which the company lowers the equipment’s price to production cost to allow as many people as possible to buy it, provide related data as subscription serve and make profits.
The company expects its subscription service will expand because it has various pipelines aside from CART, Lee noted.
“We are considering lowering the device’s price close to production cost to let many people can buy it,” the CEO said. “We will make one device be able to collect various data. As we have upcoming pipelines, including those for sleep apnea and cardiac insufficiency, the company is likely to raise its revenue through high-quality subscription services.”
In conclusion, CEO Lee stressed that his company would play the role of a bridge that links patients, hospitals and medical workers.
“So far, medical workers have treated and managed diseases mostly inside the hospitals. My hope is to link such activities outside of hospitals. Through this process, I will make Sky Labs help to create an environment to make people live healthier and longer.”

