Drugmaker denies causal relationship exposed by Lancet’s 2016 study
Could it be that Gilead’s new hepatitis B medicine Vemlidy (compound tenofovir ala fenamide, TAF) has more adverse effects than the current drug Viread, by raising urine glucose and LDL-C(low-density lipoprotein cholesterol) levels?
That is a question posed by some industry watchers about Gilead Science’s new drug, as a follow-up to Viread, which brought the revenue of 150 billion won ($121 million) to the company in 2016 alone. Gilead Sciences길리어드사이언스 was confident Vemlidy was safer than Viread through a series of researches that compared their efficacy and safety.
Included in the comparative studies of the two drugs, however, were the results indicating increased LDL-C in the Vemlidy clinical group, a symptom that had not been seen among the patients who took Viread.
One of such studies was “Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomized, double-blind, phase 3, non-inferiority trial,” released by Lancet in November 2016.
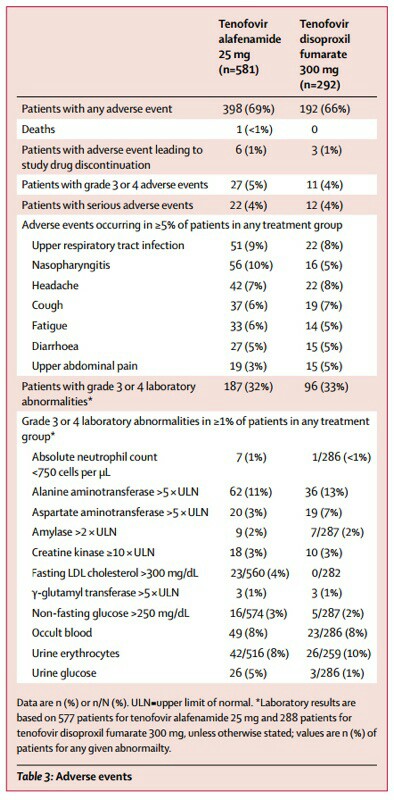
According to the study, patients who took Vemlidy베믈리디 showed higher levels of LDL-cholesterol and urine glucose than patients who took Viread비리어드 in empty stomach (Fasting LDL cholesterol >300 mg/dL(Vemlidy 4 percent, Viread 0 percent), Urine glucose(Vemlidy 5 percent, Viread 1 percent).
It conducted the research from September 2013 to December 2014 in 161 centers of 19 countries with 873 chronic hepatitis B patients (581 patients who took Vemlidy, and 292 patients who took Viread) for 48 weeks. The primary endpoint of Phase 1 clinical trial to compare Vemlidy (25mg) and Viread (300mg) was less than hepatitis B virus DNA figure 29IU/mL in blood. Gilead Sciences supported the research.
About the increase of LDL-C, Gilead said, “Patients with dyslipidemia or LDL increase showed LDL-C rise and the research has already indicated it had failed to prove a direct relationship with Vemlidy. Given the data change of HDL-C (high-density lipoprotein cholesterol) against total cholesterol change, the effect of Vemlidy was ‘neutral.’”
As to the glucose increase, it said, “The urine glucose level was higher in the Vemlidy patient's group than the opposite group because the researchers randomly assigned more patients with diabetics or high blood sugar. There is no relationship between Vemlidy and urine glucose.”
Experts said parties involved need to watch the results of the new drug’s use for a long time, however.
“It is noteworthy that the Vemlidy group showed more bone mineral density (BMD) and serum creatinine than the Viread group,” said Professor Lee Seung-won이승원at gastrointestinal internal medicine of Bucheon ST. Mary’s Hospital부천성모병원. “The increase of urine glucose level, however, makes us question whether the ‘renal protective effect’ would maintain for a long time.”
Lee went on to say, “Given the fact that hepatitis B patients are likely to take Vemlidy for a long time, LDL-C increase can be a concern. We should continue to watch whether Vemlidy can completely replace Viread whether the new drug has similar efficacy to the old one while overcoming its predecessor’s weaknesses.”
The Ministry of Food and Drug Safety (MFDS)식품의약품안전처 approved Vemlidy this month with the indication for adult chronic hepatitis B treatment to take one tablet one time a day.
In a news conference on May 17, Gilead said 25mg-Vemlidy has noninferiority antivirus effect and fewer side effects in kidney and bone mineral density than Viread. But it didn’t recommend the drug to patients with terminally-ill kidney malfunction (less than 15mL/min of creatinine clearance estimate) or cirrhosis.

