A local research team has found that it takes about 300 days for multinational pharmaceutical companies to receive approval for new drugs in Korea.
The study, led by Professor Lee Jae-hyun at Sungkyunkwan University, included 115 new drugs from 23 Korea Research-based Pharma Industry Association members licensed in Korea from 2011 to 2017. It was the first research to investigate the actual time required for companies to receive approval for new drugs in Korea.
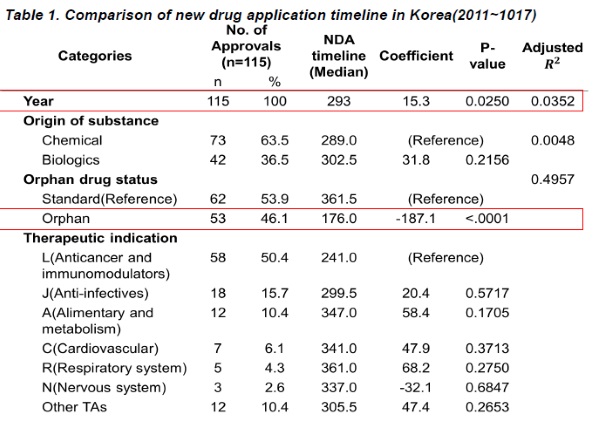
According to the report, the average approval process period for the 115 medications was 299.7 days. Although the research was unable to pinpoint any specific trends, the study did confirm that the processing time has been increasing over the past three years.
Among the new drugs, 73, or 63.5 percent, were synthetic drugs and 42 (36.5 percent) were biopharmaceuticals. The median approval process time for synthetic drugs was 289 days, while that for biopharmaceuticals was 302.5 days.
The research also confirmed that orphan drugs took less time to receive approval than non-orphan new drugs. Orphan drugs totaled 53 in number, accounting for 46.1 percent of the total, and took an average of 187.1 days to complete the approval process, compared to the non-orphan drug approval time of 361.5 days.
“Although the approval duration was similar in comparison with other developed countries, there was a big variation by item and year in Korea,” the KRPIA said after analyzing the research. “To increase the predictability of permit durations and improve the approving process, Korea should adopt a method to determine the total duration of the permit review process, including supplementary periods, similar to other developed countries.”
Also, there is a need to improve the practical administrative procedures, regulatory environment, and auditing human resource management, such as rationalizing permit requirements in line with international regulatory guidelines and the expanding staff in the audit department, the group added.