Yuhan Corp. said its investigational anticancer drug Lazertinib has proved the efficacy and safety against lung cancer in the latest multiple clinical trials.
At the virtual meeting of the American Society of Clinical Oncology (ASCO) on Friday, the company made three poster presentations on Lazertinib’s therapeutic effect against lung cancer, its effect on brain metastasis, and the findings on its clinical genetics on the mechanism of resistance after the drug administration.
Yuhan is developing Lazertinib as a targeted therapy for patients with non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutation.
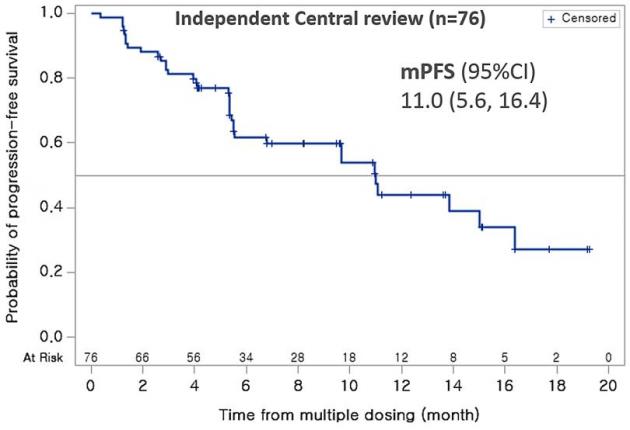
Researchers gave Lazertinib 240mg to 76 patients with T790M mutation in EGFR. The result showed that the patient’s objective response rate (ORR) by the independent central review was 57.9 percent (complete response in two patients), and the investigator-assessed ORR was 72.4 percent.
The median of progression-free survival (PFS) by the independent central review was 11 months, and the investigator-assessed one was 13.2 months. The new outcomes are an improvement from Lazertinib 240mg’s ORR at 50 percent, released at the last year’s ASCO meeting.
The most common adverse reactions included rash (35 percent), pruritus (33 percent), and paraesthesia (32 percent), which were mostly mild. Five percent of the patients discontinued the treatment due to adverse reactions.
Yuhan also published the result of the study on Lazertinib’s intracranial anti-tumor effect in NSCLC patients with brain metastasis.
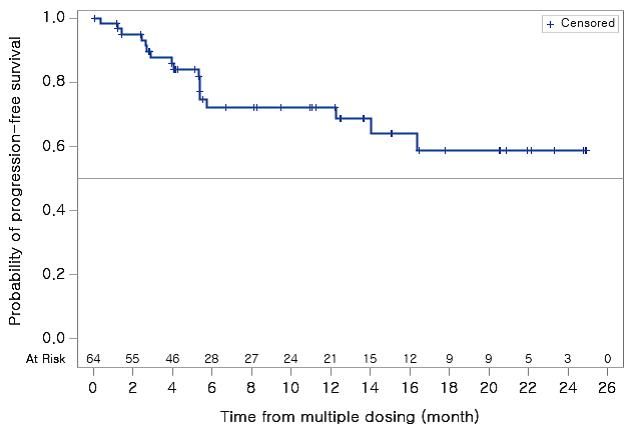
The researchers administered Lazertinib 20-320mg to 64 NSCLC patients whose tumor spread to the brain before the start of the Lazertinib treatment. They found that Lazertinib’s intracranial disease control rate (IDCR) was 90.6 percent. The 240mg group showed 16.4 months of PFS. Among the brain metastasis population evaluable for response, 22 patients’ objective intracranial response rate (OIRR) was 54.5 percent.
“This study showed that Lazertinib could help treat lung cancer with EGFR mutation, even with brain metastasis,” said Kim Sang-we, the lead author of the study and a professor at the Oncology Department of Asan Medical Center.
The company also released the results of the study on how 47 patients developed a genetic mutation that led to their disease progress after the Lazertinib treatment. The researchers found that the most common resistance mechanisms were T790M loss and PIK3CA alterations. The researchers said the spectrum of the mechanism of resistance identified in the patients treated with Lazertinib was similar to that reported in other third-generation EGFR-targeting therapies.
Yuhan said it planned to recruit patients for a phase-3 trial on Lazertinib as the first-line treatment. The company is also testing the combination of Lazertinib with amivantamab, an experimental bispecific antibody therapy by Janssen.

