Last month, a parliamentary audit on the Ministry of Food and Drug Safety revealed that some people sold and purchased prescription medicines illegally on a secondhand marketplace Danggeun Market using mobile phones. Three weeks later, however, illicit practices are still ongoing.
People were selling and purchasing steroid ointment, reflux esophagitis treatment, SGLT-2 inhibitors for diabetes, and even hair loss treatment.
Under the Pharmaceutical Affairs Act, people other than pharmacists or Oriental medicine pharmacists cannot sell pharmaceutical products. The law even bans pharmacists from selling prescription drugs without a doctor’s or dentist’s prescription and prohibits purchasing illegally traded prescription drugs.
Korea Biomedical Review (KBR) has tried purchasing a prescription drug directly from a seller in the Danggeun Market to determine how the illicit drug deals were made and how the market restricted such deals.
Steroid ointment purchased in just 5 minutes
On the mobile application of Danggeun Market, many users sold steroid-using dermatological prescription drugs, including Titibe Cream and Desowen Cream.
Steroid ointments are potent, but they can cause side effects such as contact dermatitis and folliculitis. However, the app's sale postings did not state that the drugs required a doctor’s prescription. They did not even mention that the treatments contained steroids.
The seller of Titibe Cream, categorized as Grade 5 in the scale of seven grades with Grade 7 the weakest in steroid ointment, uploaded the sale posting three weeks before the KBR’s purchase attempt. However, the market has not taken any measure on the posting for the past three weeks.
KBR contacted the ointment seller through the chat room inside the Danggeun Market app. It took only 12 minutes to purchase the prescription drug. The seller expressed the intention to sell the treatment in just five minutes after receiving KBR’s contact and gave the location where KBR could pick up the product.
Receiving the ointment and paying cash, KBR asked the seller if it would be okay to buy it without a doctor’s prescription. The seller seemed to be confused a little but did not explain further or stop the deal. Instead, the seller explained that the treatment could be applied once or twice a day, as written on the drug package.
Kim Hyun-jung, a professor at the Dermatology Department of Chungnam National University Sejong Hospital, warned that people should use steroid ointment very carefully because even physicians prescribe a different ointment for each different site of the body.
“Abuse of steroid cream without a prescription can cause tinea incognito, a fungal disease, and rosacea, a swelling skin disease with redness in the face,” she said.
She went on to say that side effects after a drug use without a prescription make it difficult for a doctor to correct the problem because doctors normally look into the history of prescriptions to switch to another drug or adjust the dosing.
Using a steroid ointment without a prescription can make it hard for a doctor to identify the problem's cause, she said.
Illegal deals of diabetic drugs, hair loss treatment already made
In the Danggeun Market, people were selling prescription medicines regardless of the severity of the disease and the purchasers' and sellers' location.
KBR has found over 30 prescription treatments, either in progress for sale or already sold, in Danggeun.
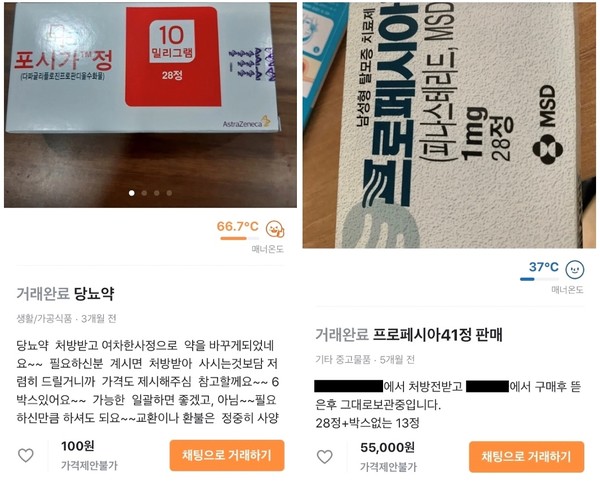
More specifically, the illegally traded medicines included antidiabetic drug Forxiga, hair loss treatment Propecia, obesity drug Lipidown Cap., morning sickness relief pill Diclectin Enteric Coated Tab., hyperkalemia treatment Kalimate Powder, arthritis drug Joins Tab., expectorants Synature Syrup and Duphalac Syrup, and reflux esophagitis treatments Storin Liq. and Algin N Solution.
Forxiga, an SGLT-2 inhibiting diabetes drug, poses a risk of fetal malformation and fetal toxicity if taken by a pregnant or possibly pregnant patient. The drug’s risk required so much attention that the U.S. FDA issued a warning in 2018 to notify the risk of serious infections around the genital tract when using SGLT-2 inhibitors.
Propecia is also banned for pregnant people. In 2012, the government ordered changes in approval conditions and issued a warning on the use, following the FDA’s reports of finasteride-containing drugs’ sexual function-related side effects after the administration.
However, the sellers in Danggeun did not mention the risk of using prescription drugs without a doctor’s permission.
If people trade prescription drugs through a secondhand market, they will face the storage problem. Some medications might need to be stored in a refrigerator, and there is no guarantee whether the drugs were within the expiration date.
If it is impossible to verify a prescription drug's storage status, it means that the person who bought the drug is taking the medication without the safety guarantee.
Professor Kim Dae-jung of the Endocrinology and Metabolism Department of Ajou University Hospital said a secondhand drug deal was a “very wrong idea.”
“The act does not consider the problem of the wrong use or abuse of a drug,” he said.
Report, ban, cut of illegal drug deals nowhere to be found
At the National Assembly’s audit on Oct. 13, Danggeun Market CEO Kim Jae-hyun and former Food and Drug Safety Minister Lee Eui-kyung said they would take countermeasures against the illegal drug sales and purchases in the Danggeun Market.
Kim said the company has been blocking illicit drug deals through user reports and filtering function, but the service has not been sufficient due to increased users and transactions.
He said the company would address technical problems to prevent similar incidents.
Lee said the government would enhance monitoring on secondhand drug deals and block the sellers and purchasers' access.
However, when KBR tried purchasing a prescription drug, it did not receive any warning or reprisal from Danggeun Market until it successfully completed the deal.
In response to KBR’s inquiry, Danggeun Market said, “We have been filtering out problematic items by keywords based on the list of drugs provided by the food and drug safety ministry since September.” The company enhanced the keyword filtering through self-monitoring and reporting by users, it added.
Danggeun Market said it notified users that pharmaceutical products are banned from the secondhand market on its website, social media, and in-app notification.
The app operator also individually notified the sellers of drugs selling prohibited items and kicked them out of the secondhand market if they posted such illegal items repeatedly.

