Multinational pharmaceutical companies expanded their R&D investment in Korea by 20 percent on-year to 700 billion won ($499.5 million) in 2021, their representative group said Monday.

On Monday, the Korea Research-based Pharma Industry Association (KRPIA) published a report on the results of a survey on R&D expenses and research manpower from 31 offshoots of global pharmaceutical companies operating in Korea.
The study also included improvement measures and activities contributing to domestic R&D development, including R&D costs invested by global pharmaceutical companies, human resources, and the status of various clinical studies as of 2021.
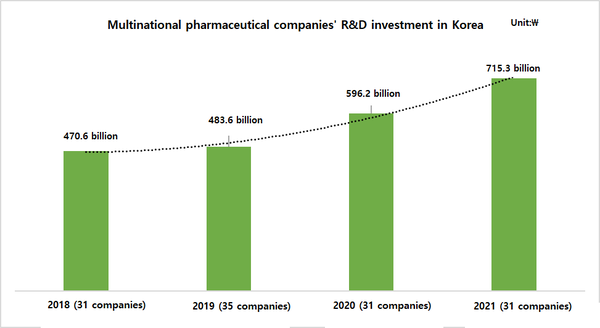
According to the report, the total R&D cost invested in clinical research by global pharmaceutical companies in 2021, excluding R&D costs directly outsourced by the overseas headquarters, was about 715.3 billion won, a 19.96 percent increase from the previous year.
By number, a total of 1,590 clinical studies were conducted last year, an increase of about 6.1 percent compared to the previous year. The figure has steadily increased over the past four years.
The proportion of clinical trials for anticancer drugs was the highest with 66.3 percent.
The KRPIA report also showed that global pharmaceutical companies are expanding their domestic clinical trial infrastructure to provide new, high-quality treatment opportunities for Korean patients.
A total of 16,342 patients participated in phase 1, 2, and 3 clinical trials, an increase of about 24 percent from the previous year.
Notably, the report outlined that the participation and contribution opportunities of Korean researchers were expanding to the initial stage of new drug development as the rate of increase in phase 1 and 2 trials in 2021 rose significantly compared to the rate of increase in late phase 3 trials in 2021.
“Global pharmaceutical companies are expanding the new treatment options to Korean patients, ranging from severe and rare intractable diseases, which are difficult to treat with existing medicines, through continuous R&D investment, such as expanding clinical trial infrastructure and nurturing excellent human resources in Korea,” a KRPIA official said. “
However, despite the increase in Korean clinical trials for new drugs, the KRPIA official stressed that the adoption rate of new drugs in Korea was still low.
“To increase access to cutting-edge new drugs for Korean patients, Korea requires institutional and policy improvements in determining approval, reimbursement, and drug prices,” the KRPIA official said. “Such improvements are necessary to maintain R&D investment in Korea.”
Related articles
- ‘Rare disease patients still have poor access to treatment’
- KRPIA raises concern over HIRA’s cost-effectiveness evaluation plan
- Multinational drugmakers’ R&D in Korea grew for 5 consecutive years
- Korea’s drug approval too slow, compared to U.S., Europe: KRPIA
- Multinational pharmas' R&D in Korea up 14% in 2022: KRPIA
- KRPIA applauds agreement between Korea, EU to enhance medicine information sharing

