GC Pharma said on Monday that its neutropenia treatment, Neulapeg (ingredient: pegteograstim), confirmed effectiveness in a phase 2 study on patients with recurrent and refractory multiple myeloma.
Neulapeg is a second-generation neutropenia treatment, which is an anticancer adjuvant that prevents hematological side effects of reduced immunity due to a decrease in neutropenia levels in the body when anticancer drugs are administered to patients.
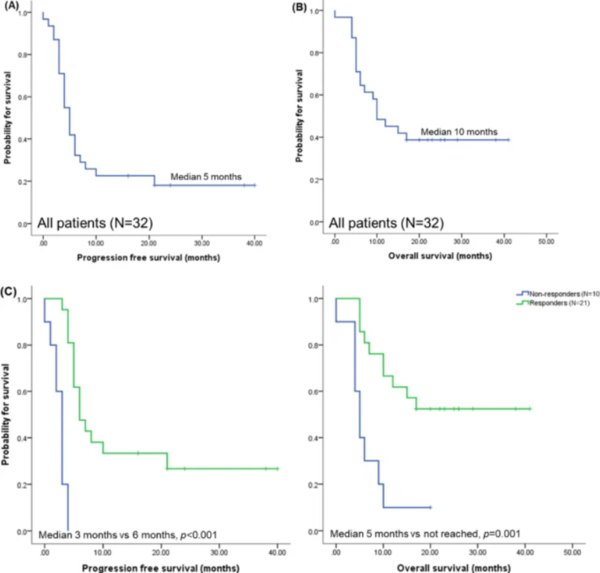
The study analyzed the impact of Neulapeg on reducing anticancer and hematological side effects of daratumumab (anti-CD38 monoclonal antibody) in combination with dexamethasone, cyclophosphamide, etoposide, and cisplatin (DCEP) in 32 patients who did not respond to or recurred after the primary treatment of bortezomib.
Upon evaluating grade 3 or higher neutropenia for each chemotherapy cycle, grade 3 or higher neutropenia (absolute neutrophil count fewer than 1,000) was found to be 8.0-13.8 percent. This marks a significant reduction compared to prior studies which cited 35.5-91.5 percent neutropenia of grade 3 or higher when daratumumab and DCEP monotherapy were applied respectively.
When neutropenia of grade 3 or higher occurs, chemotherapy is often delayed or administered at a lower dose, which adversely affects the patient's prognosis.
Additionally, the objective response rate (ORR) of the combination therapy was 67.7 percent, which significantly improved compared to the monotherapy of daratumumab (ORR 29.2 to 42.1 percent) and DCEP (ORR 44.4 percent).
Overall, Neulapeg improved the ORR of daratumumab-DCEP combination therapy because it was possible to proceed as planned with chemotherapy by administering Neulapeg from the first anticancer cycle to prevent neutrophil reduction.
Professor Koh Young-il of Seoul National University Hospital said, "Through this study, prophylactic administration of Neulapeg in those at risk of developing neutropenia from chemotherapy, improved both the patients quality of life and their prognosis."
According to data from IQVIA, Neulapeg accounted for 49 percent of the neutropenia treatment market last year, making it the most prescribed second-generation neutropenia treatment.
The study was published in the latest issue of the Journal of Hematology & Oncology.

