In early 2018, AstraZeneca proved Tagrisso (osimertinib)’s efficacy in improving the progression-free survival (PFS) of EGFE-mutation non-small cell lung cancer as its primary treatment based on a global phase 3 clinical trial of FLAURA study.
Tagrisso also demonstrated its benefits in overall survival (OS), solidifying its position as the global gold standard in primary treatment in early 2020.
However, the drug could not become a standard treatment in Korean clinical fields due to its failure to improve OS in the L858R patient groups and the Asian cohort. The Ministry of Food and Drug Safety gave the nod in 2019, but the drug remains out of insurance benefits, a critical threshold to becoming a standard therapy.
Against this backdrop, some participants in the ESMO (European Society for Medical Oncology) Asia 2022 on Dec. 2-4 in Singapore released real-world study results on Tagrisso’s primary treatment in various clinical fields.
The real-world data on Japanese patients, who had brought about OS failures in the Asian cohort in the FLAURA study, drew particular attention at the conference.
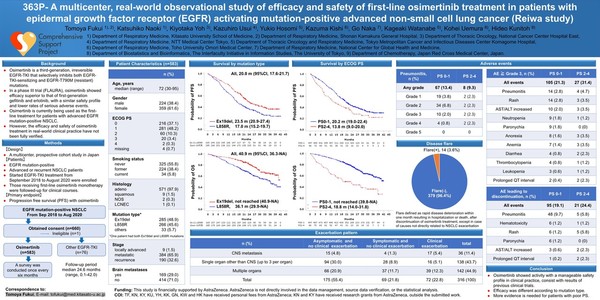
The Japanese patients who participated in the study accounted for the largest share of Asians. However, the Japanese patients differed from global patients because many were diagnosed as early-stage patients, not the fourth-stage, and experienced recurrence after receiving surgery. Besides, the share of L858R mutation patients with poorer prognoses was also high in Japanese patients.
In addition, the Japanese cohort group had a loophole because a higher share of patients discontinued Tagrisso administration in the initial stage. Accordingly, one could not be certain the final OS data was due to Tagrisso or other EGFR (epidermal growth factor receptor) TKIs (tyrosine kinase inhibitors) used in the follow-up treatment.
These problems worked as biases against the Japanese cohort, adversely affecting the Asian cohort, according to many Korean experts.
However, the latest real-world data of the Japanese patients exactly reproduced the survival benefits demonstrated by Tagrisso in the FLAURA study.
The mPFS (median value of PFS) of 583 patients who received the primary treatment from 2018 to 2020 was 20 months, similar to 18.9 months in the FLAURA study.
Tagrisso group’s mOS (median overall survival) in the median point of the 24.6-month-long tracking observation period was 40.9 months. That of L858R patients was 36.1 months, and Exon 19 defective mutant patients have failed to reach the median value. It was also similar to the mOS of 38.6 months shown in the FLAURA study.
“Based on the 2018 FLAURA study, Tagrisso simultaneously won the nod and insurance benefits in Japan, too. It is now recommended as a standard treatment in a guideline, and 88 percent of Japanese received the primary treatment with Tagrisso in the real-world environment,” said Dr. Tomoya Fukui at Shonan Kamakura General Hospital, who released the study result. “The recent real-world study was significant as Tagrisso displayed anticancer activity and a manageable safety profile consistent to the FLAURA study results in actual clinical fields.”

Professor Frank Griesinger, head of the Hematology-Oncology Department at Pius-Hospital Oldenburg, Germany, also draws attention to his real-world study results in his country.
Professor Griesinger analyzed the data of 217 patients treated with Tagrisso from June 2018 to December 2020 in Germany and found that the mPFS was 16.2 months, shorter than the FLAURA study.
However, the German experts said the result was due to basic differences of patients in an interview with Korea Biomedical Review on the sideline of the conference.
“It is difficult to make a collective comparison of PFS figures of the real-world analysis with clinical studies,” Dr. Griesinger said. “In the pertinent study, the share of participants with central nerve system metastasis was higher at about 38 percent, and it also included uncommon mutant patients (11 percent). Besides, the patient’s average age was about four years higher.”
The German expert said, “In conclusion, we could interpret that the recent study consistently confirmed Tagrisso’s primary treatment effects demonstrated in the FLAURA study.”
Professor Griesinger expressed particular concerns over the sequential treatment strategy – where patients use first- and second-generation drugs and then the third-generation drug later – in Korea. He then explained why not all patients could use third-generation drugs in follow-up treatment.
“Only 70-80 percent of patients with advanced diseases can undergo a mutation test,” he said. “Basically, in patients who have already developed resistance, some do not have tissues left to perform a biopsy.”
He then cited recent data that said only 38 percent of patients receive biomarker tests after developing resistance in Asia.
Not only can all patients undergo the test but only about half of the patients who undergo the test can confirm T790M positivity. Not all patients can use Tagrisso as secondary treatment,” Professor Griesinger said.
Moreover, 20-30 percent of patients die before they reach the second treatment stage, having no opportunities for secondary treatment, the German clinician said, emphasizing one must take this into account.
“Compared to first- and second-generation TKIs, Tagrisso has better drug tolerance and shows excellent efficacy in brain metastasis. More than anything else, it has the data of improving OS,” Professor Griesinger said. “In Germany, more than 90 percent of patients receive primary treatment with Tagrisso, which has taken root as the standard treatment. All German guidelines also recommend primary treatment with Tagrisso.”
Meanwhile, AstraZeneca recently submitted an application for Tagrisso’s insurance benefits to the Health Insurance Review and Assessment Service as the primary treatment for EGFR-mutation NSCLC.
Related parties watch whether Korean patients will be able to receive the global standard treatment accompanied by insurance coverage in 2023 after four years of postponement.

