Following Roche’s Tigagolumab, Gilead’s anti-TIGIT antibody candidate substance, Domvanalimab, has also shown consistent survival benefits when combined with anti-PD (programmed cell death)-(ligand) 1 antibody in phase 2 clinical trials.
T-cell immunoreceptors with immunoglobulin and ITIM domains (TIGIT), an immune checkpoint receptor, can suppress T-cell activation and promote T-cell exhaustion. Conversely, inhibition of TIGIT may increase cytotoxic T-cell proliferation and function.
In the Plenary Series of the American Society for Clinical Oncology (ASCO) on Tuesday (local time), the results of the ARC-7 study, the phase 2 clinical trial of Domvanalimab, were unveiled for the first time.
In the ARC-7 study, researchers compared and evaluated the treatment effectiveness of three therapies on 150 patients with progressive non-small cell lung cancer who had had no treatment experiences with 50 percent or more PD-L1 TPS – PD-1 antibody Zimberelimab monotherapy (Z hereafter), two-drug combination therapy of Domvanalimab + Zimberelimab (DZ hereafter) and the three-drug combo therapy of Etrumadenant, A2a/A2b receptor antagonist) + Domvanalimab + Zimberelimab (EDZ).
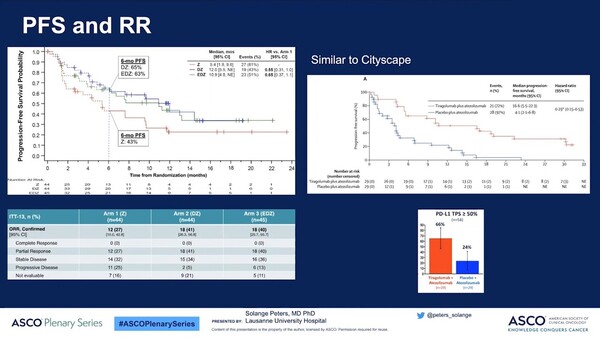
The effectiveness result disclosed this time around included 133 people’s data. During about one-year-long observation period, the objective response rate (ORR) was 27 percent, 41 percent, and 40 percent in the three groups, respectively, confirming the improved response rates in the two- and three-drug combo therapy groups.
Also, disease progression and death risks were reduced statistically significantly in the DZ and EDZ-administered groups compared to the Z group. At collecting data, progression-free survivals were 5.4 months, 12 months, and 10.9 months in the Z, DZ, and EDZ groups. Their progression-free survival rates at the six months were also 43 percent, 65 percent, and 63 percent.
Treatment-related adverse effects with third degrees or higher occurred in half the patients of each group. However, the DZ group showed no increases in immunity-related ill effects.
“Single immunotherapy is currently the standard treatment in many phases 4 NSCLC patients. However, only a small number of patients can enjoy real benefits in the long term,” said Dr. Melissa Johnson at the Sarah Cannon Research Institute, who presented the result. “Accordingly, it is important to find new combination therapies to improve their treatment results.”
Dr. Johnson added that as proved in the stable disease (SD) and partial response (PR), she was encouraged by the number of patients receiving benefits in the sixth month. “The recent result was an interim analysis, whose data will keep maturing through longer-term follow-up measures,” she said.
The benefits of Domvanalimab combination therapy, similar to the results shown by the combined use of anti-PD-L1 antibody Tecentriq (atezolimumab) in the CITYSCAPE study, the phase 2 clinical trial of Roche’s Tiragolumamb, have made the hypothesis that the simultaneous inhibition of TIGIT and PD-(L)1 could create synergic effects as a fact.
In the CITYSCAPE study, researchers are comparing and evaluating the combination therapy of Tiragolumab + Tecentriq with placebo + Tecentriq on phase 4 NSCLC patients with no previous treatment experiences and PD-L1 TPS of 1 percent or more. According to a predefined exploratory analysis, the Tiragolumab + Tecentriq combination therapy groups reduced disease progression and death risks by 71 percent in the patient group with a high PD-L1 expression rate compared to the Tecentriq monotherapy group.
In the exploratory analysis, the median value of Progression-Free Survival (mPFS) was 16.6 months and 4.1 months in the Tiragolumab + Tecentriq and Tecentriq monotherapy groups, and the former also showed significant improvement in ORR, with 69 percent vs. 24.1 percent.
Meanwhile, according to presentations at the debate session, in addition to the anti-TIGIT antibody candidate substances – Domvanalimab (ARC-10 study) and Tiragolumab (SKY-01 study) -- that are conducting phase 3 trials for the primary treatment of NSCLC using combination therapy with PD-(L)1 antibody drugs, there are also MSD’s vibostolimab (KEYVIBE-003 study) and BeiGene’s ociperlimab (AdvanTIG-302 study), heralding a fierce competition among TIGIT inhibitors as early as from 2025.

