A group of Korean researchers has found a clue to treating rheumatoid arthritis, an autoimmune disease.
The researchers, led by Professor Park Seong-ho of the Life Science Department at Ulsan National Institute of Science and Technology (UNIST), said Tuesday they have discovered a mechanism involved in the formation and differentiation of osteoblasts that damage joint bones in rheumatoid arthritis patients.
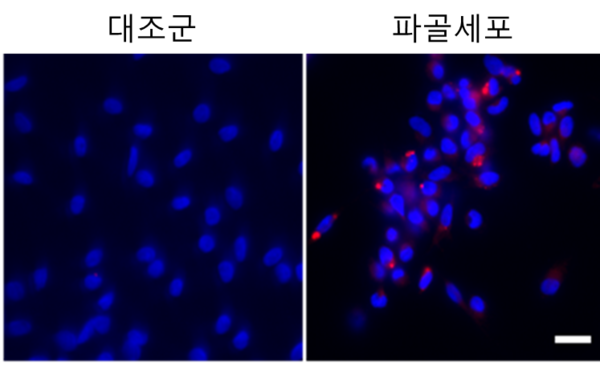
The research team studied a treatment method that targets mechanisms related to differentiating osteoblasts that melt bones by enzyme reactions.
As a result, it found posterior genetic modulation called the “superenhancer” near the NFATC1 gene, which is an important factor influencing osteoclast formation.
An enhancer refers to the part located far away from its genes in a DNA sequence and is involved in regulating gene expression. Superinshancer has a structure where enhancers gather at high density and have strong cell system specificity and activity.
NFATC1 superenhancer was formed only in osteoblasts, not appearing in other cells related to rheumatoid arthritis.
As a result, enhanced RNA, a type of NFATC1 superenhancer non-coding RNA, was formed. Unlike mRNA, which encodes proteins, non-coding RNA is an RNA molecule that does not contain information on the sequence of protein amino acids or produce proteins. Instead, non-coding RNA has the function of regulating genes related to it. The research team explained that it could be easily targeted for treatment due to molecular sequence specificity.
The research team confirmed that the disappearance of NFATC1 superenhancer RNA also inhibits the osteoblast differentiation process. Therefore, the superenhancer RNA formed in osteoblast differentiation could be used as a therapeutic target.
“The study result will make great progress in developing rheumatoid arthritis,” Professor Park said.
Their research paper, “RANKL-responsive epigenetic mechanism reprograms macrophages into bone-resorbing osteoclasts,” will be published in the January issue of Cellular and Molecular Immunology.

