Lunit said it has completed validation of Lunit Scope PD-L1, its artificial intelligence pathology analysis solution, in Clinical Laboratory Improvement Amendments (CLIA)'s laboratory-developed test (LDT).
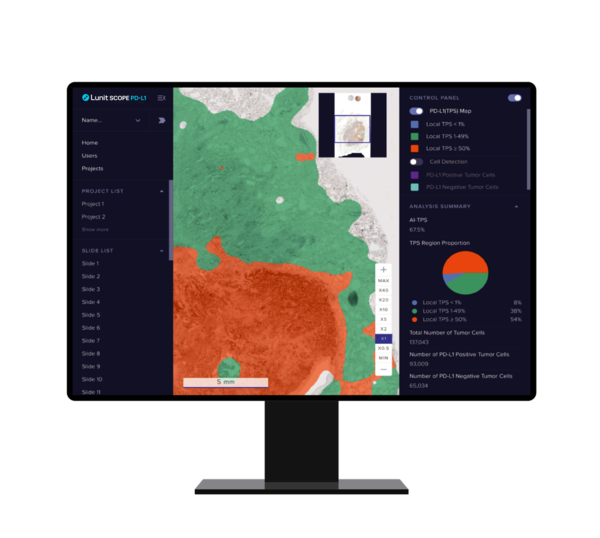
The company conducted the test at Guardant Health's CLIA Lab. According to the joint research and development contract between the two companies, Lunit received 1.3 billion won ($1 million) as a milestone payment from Guardant Health.
CLIA is a certification given by the Center for Medicare & Medicaid, which operates the U.S. national health insurance system, deciding whether the environmental level required for diagnosis meets the U.S. laboratory standards. By acquiring CLIA certification, companies can sell their products and services in the U.S. market.
LDT is a system that allows CLIA-certified laboratories to provide self-developed diagnosis and inspection services.
Lunit underwent the LDT validation to expand clinical testing to non-small cell lung, triple-negative breast, and bladder cancer. It also plans to complete the CLIA LDT verification for 10 other cancer types within the first half of this year.
However, the company did not specify which cancer types it plans to verify.
According to Lunit, the recent LDT verification is an extension of the additional development of Guardant360 TissueNext, recently released by Guardant Health in collaboration with Lunit.
Guardant360 TissueNext is a product that accurately and objectively analyzes the expression level of PD-L1 (Programmed death-ligand 1), a protein on the surface of cancer cells, and a biomarker that predicts the treatment efficacy of immunotherapies.
"Lunit took its technological competitiveness to the next level by completing the CLIA LDT verification, following the release of the first new product in the field of cancer treatment with Guardant," Lunit CEO Suh Beom-suk said.
As Guardant has secured a large-scale distribution and sales channel in the U.S., Lunit expects to expand its presence in the market, Suh added.
Related articles
- Lunit to showcase novel cancer diagnostic platforms at AACR 2023
- Indica Labs, Lunit forge strategic alliance for integrated digital pathology AI workflow
- Lunit sales in Q1 skyrocket over threefold compared to 2022
- Guardant Health launches blood test for colorectal cancer in Korea
- Guardant Health's blood test for colorectal cancer screening wins FDA approval

