Keytruda (pembrolizumab), which lost the market leadership to Opdivo (nivolumab) in gastric cancer treatment due to the failure of the KEYNOTE-062 study in 2019, has made a splendid comeback with the success of the KEYNOTE-859 study.

Professor Rha Sun-young of Yonsei Cancer Center conducted a successful clinical trial, demonstrating Korea’s status once again in gastric cancer treatment worldwide.
Prof. Rha released the first analysis result of the KEYNOTE_859 study in the recent European Society for Medical Oncology (ESM) Virtual Plenary session.
KEYNOTE-859 was a phase 3 clinical study that compared Keytruda + chemotherapy with the placebo + chemotherapy in the primary treatment of 1,579 patients with HER2- locally progressive or metastatic gastric/gastroesophageal junction cancer.
In the previous KEYNOTE-062 study, Keytruda compared its monotherapy or combination therapy with chemotherapy alone. Still, both groups failed to produce statistically significant overall survival (OS), and progression-free survival (PFS) compared to the control group, according to the study results released in 2019.
Four years later, Keytruda confirmed its clinical value in HER2- gastric cancer patients through the KEYNOTE-859 study.
According to Professor Rha’s presentation, Keytruda added to chemotherapy showed statistically noticeable and clinically significant improvement in OS, PFS, and objective response rate (ORR) compared to chemotherapy alone.
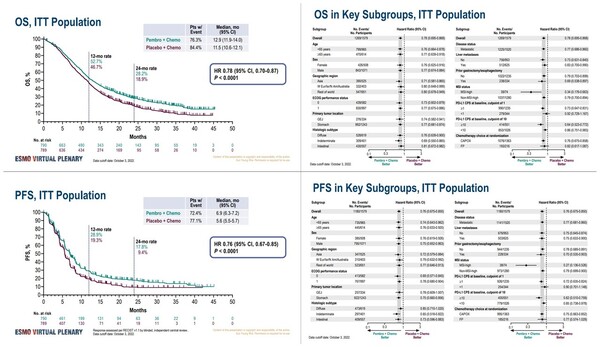
In the primary endpoint of median OS (mOS), the Keytruda-added group was 12.9 months, and the control group was 11.5 months. The two-year survival rate was 28.2 percent vs. 18.9 percent, with the Keytruda showing a 22 percent lower death risk than the control group.
The secondary endpoint of median PFS (mPFS) was 6.9 months against 5.6 months, and the two-year PFS was 17.8 percent vs. 9.4 percent, with the Keytruda-added group showing 24 percent lower disease progression and death risks.
The advantages in OS and PFS were consistent in all subgroups, differentiated by regions, PD-L1 expression rates, and chemotherapy types. The additional administration of Keytruda also improved the response rate (with an ORR of 51.3 percent against 42.0 percent) and the duration of response (DoR) (with median DoR (mDoR) of 8.0 months against 5.7 months).
‘Big stride for phase-4 HER2- gastric cancer patients with poor prognoses’
In a recent interview with Korea Biomedical Review, Professor Rha evaluated the success of the KEYNOTE-859 study as “a big stride for phase-4, HER2- gastric cancer patients with poor prognoses.”
Following Opdivo, Keytruda has also proved improved survival by combining with chemotherapy, cementing its value as the immunotherapy that can offer an option as the standard treatment of phase-4, HER2- gastric patients, she said.
“The KEYNOTE-859 study also used ‘22C3 anti-PD-L1 antibody to determine the expression rate of PD-L1,” Rha said. “In other words, we assessed its function using more valid criteria of ‘CPS 1’ and ‘CPS 10.’”
Such clinical design can make jobs easier at hospitals and pathological departments when they use Keytruda in clinical fields after the new treatment wins approval.
“Currently, most Korean hospitals are equipped with 22C3 diagnostic devices, which are already used in other cancers,” Rha said. “In addition, CPS 1 and 10 are reproducible and valid criteria at all pathological departments, free from concerns about the sensitivity issue.”
However, Keytruda’s advantage is also Opdivo’s disadvantage.
Opdivo’s CheckMate-649 study used a “28.8” antibody to determine patients’ PD-L1 expression rate and presented CPS 5 criteria. However, such a clinical trial can pose a stumbling block in using Opdivo in Korea.
Hospitals will find it burdensome to introduce equipment to diagnose "28.8" antibodies that are rarely used otherwise, and it will also be difficult for pathological doctors to diagnose CPS "5" precisely.
Nevertheless, Opdivo has been the only immunotherapy option for the primary treatment of phase-4 HRR2- gastric cancer patients.
In June 2021, Opdivo won approval as the primary treatment of progressive or metastatic gastric adenocarcinoma, gastroesophageal cancer, and esophageal cancer combined with fluoropyrimidine-family or platinum-based chemotherapies. The Health Insurance Review and Assessment Service (HIRA) also examines it for insurance benefits.
Opdivo’s insurance coverage will likely be restricted to patients with “PD-L1 CPS 5 or higher,” as noted earlier. That’s because the result of the CheckMate-649 study showed the effects of Opdivo combo therapy were significantly higher in patients with PD-L1 CPS of 5 percent or more.
MSD plans to expand Keytruda’s indication (based on the KEYNOTE-859 study) in treating gastric cancer late this year or early next year. According to the company’s plan, its competition with Opdivo will likely start in mid-023 or the latter half-year.
Professor Rha explained that if Keytruda gets domestic approval and insurance benefits, its criteria will include patients with “CPS1 or higher” or “CPS10 or higher.”
Rha added that the accompanying diagnostic devices differ in their clinical trials, and the presupposed CPS levels differ. Therefore, insurance benefits will highly likely be based on their different clinical designs.
She also expressed the hope that Keytruda’s criteria for insurance benefits will be set at “CPS 1 or higher.”
Rha pointed out that the government may want to restrict the number of patients using immunotherapies out of concerns about the health insurance’s finance. Still, the number of phase-4 gastric cancer patients who can use Opdivo or Keytruda is small.
“The prevalence of gastric cancer is on a slide in Korea, and most patients are diagnosed and cured thanks to the national cancer screening program,” Rha noted. “After all, phase-4 patients account for less than 6 percent of total gastric cancer patients.”
She went on to say, “Even among the small number of phase-4 patients, the share of patients with CPS 1 or higher accounts for about 65 percent of HER2- patients. Considering the five-year survival rate of phase-4 gastric cancer patients, it seems necessary to provide insurance benefits for patients with CPS1 or higher when they use Keytruda.”
Professor Rha noted that there had been considerable progress in treating phase-4 gastric cancer patients, such as targeted agents for HER2+ patients, immunotherapies for HER2- patients, and the appearance of claudin target antibodies, such as zolbetuximab.
“The more weapons we can use, the more helpful for treating patients,” she said. “In this regard, the success of the recent Keytruda clinical trial is a welcome development
Related articles
- Attenuated salmonella engineered to improve chemotherapy permeability
- Opdivo combo expands indication for primary treatment of metastatic ESCC
- Proteogenomic data show new therapeutic targets for intractable bile duct cancer
- MSD reviews Keytruda’s accomplishments on 1st anniversary of expanded reimbursement
- Opdivo to get reimbursement soon as primary treatment of stomach cancer

