Novo Nordisk Korea said it received the Ministry of Food and Drug Safety’s approval for Wegovy Prefilled Pen (ingredient: semaglutide) on Thursday last week.
Wegovy, a once-weekly treatment for obesity, is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management, including weight loss and weight maintenance, in adult patients.
The approval was based on STEP, a large clinical trial designed to evaluate the efficacy and safety profile of Wegovy.
In the multinational study of 1,961 adults who were overweight or obese, 1,306 patients in the Wegovy arm demonstrated a mean weight loss of 14.9 percent from baseline over 68 weeks, compared to 2.4 percent in 655 patients in the placebo group.
All overweight or obese patients in the trial participated in a reduced-calorie diet combined with increased physical activity.
Wegovy can be used in obese patients with an initial body mass index (BMI) of 30 kg/m2 or greater or in overweight patients with one or more weight-related comorbidities and a BMI of 27 kg/m2 or greater but less than 30 kg/m2.
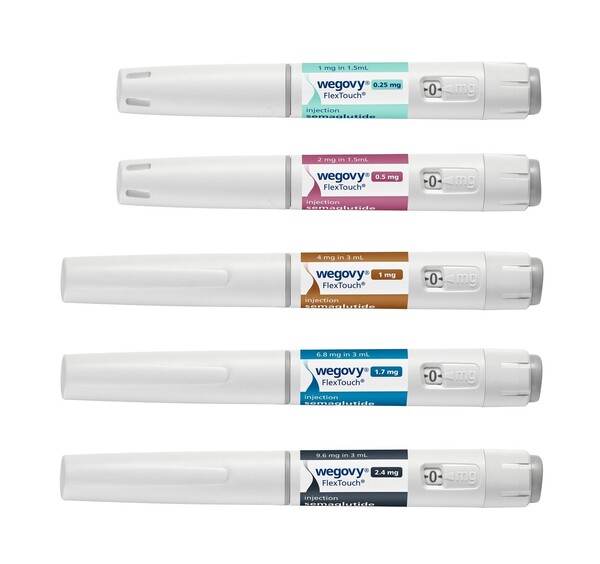
The initial dose of Wegovy is 0.25 mg once weekly and is titrated up to a maintenance dose of 2.4 mg once weekly for 16 weeks. Wegovy is available in five doses: 0.25 mg, 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg.
"Wegovy has proven its clinical utility through extensive clinical trials, and we hope it can make a difference in the lives of the 15 million South Koreans affected by obesity," said Sasha Semienchuk, general manager of Novo Nordisk Korea.

