Researchers from Yonsei Cancer Center discovered a three-drug combination -- comprising HER2-targeted therapy, trastuzumab, and ramucirumab plus paclitaxel, the existing standard of care -- which demonstrated superior efficacy and fewer side effects than other agents in patients with HER2-positive advanced gastric cancer who had failed first-line treatment.

HER2-positive gastric cancer has a poorer prognosis than HER2-negative gastric cancer because the receptor on the surface of the cancer cells causes cancer cells to divide rapidly. Currently, HER2-positive gastric cancer accounts for 10-15 percent of all advanced gastric cancers.
In the first-line treatment of HER2-positive gastric cancer, the combination of the HER2-specific targeted therapy trastuzumab and cytotoxic chemotherapy has significantly increased survival and has become the standard of care. However, second-line treatment options for patients with HER2-positive advanced gastric cancer after first-line treatment failure are limited.
The study, led by Professor Rha Sun-young from the Department of Medical Oncology at Yonsei Cancer Center, demonstrated a 54 percent overall response rate (ORR) and a 96 percent disease control rate.
Furthermore, the median progression-free survival (PFS) and overall survival (OS) were 7.1 months and 13.6 months.
The team also compared the outcomes of patients who received ramucirumab plus paclitaxel as a second-line treatment after first-line therapy versus the outcomes of the three-drug combination.
The results showed that the objective response rate (ORR) of 29 percent and disease control rate of 78.6 percent was lower in the standard-of-care group compared to those in the three-drug combo group. Similar studies such as the GATSBY and T-ACT clinical trials also showed lower ORR of 20.6 percent and 33.3 percent respectively.
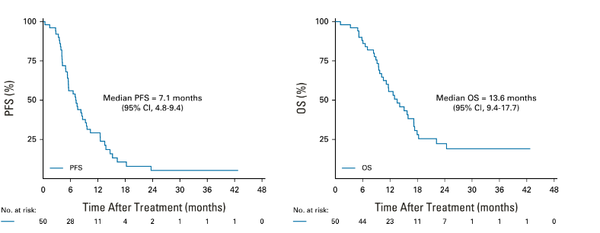
PFS and OS were also improved by 2.4 and 3.8 months, respectively, in patients receiving the three-drug combination. In particular, there was no additional drug resistance or additional toxicity compared to the standard of care, indicating a safety profile that can be used in real-world practice.
"The addition of HER2-specific targeted therapies may provide a promising second-line treatment strategy for patients with HER2-positive advanced gastric cancer who have failed conventional first-line therapy," Rha said. "We look forward to providing patients with improved treatment options in the future."
The study was published in the Journal of Clinical Oncology, an American Society of Clinical Oncology (ASCO) Journal, on June 26.

