Korean drugmakers will likely intensify marketing activities for their biosimilars of AbbVie’s autoimmune disease treatment Humira (adalimumab) this year, government and business officials said on Monday.
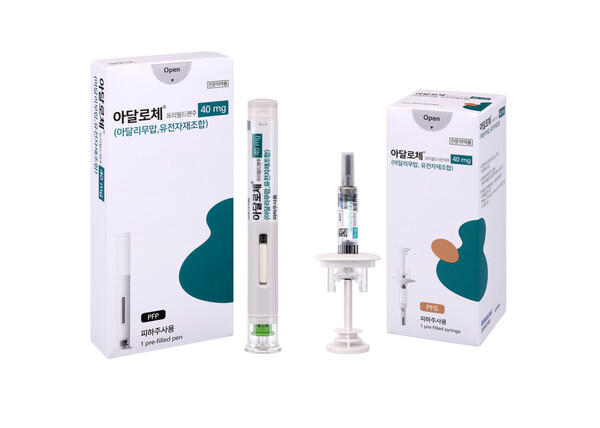
According to the Ministry of Food and Drug Safety (MFDS), Samsung Bioepis has decided to focus on supplying only the high-concentration formulation of its Humira biosimilar, Adalloce, in Korea.
Samsung Bioepis recently reported to the MFDS that it would stop supplying Adalloce's low-concentration formulations, Adalloce Prefilled Syringe Inj 40mg/0.8mL and Adalloce Prefilled Pen Inj 40mg/0.8mL. The company cited the launch and supply of a higher-concentration product with the same active ingredient as the reason for suspending the low-concentration formulations.
As a result, Samsung Bioepis will only supply Adalloce's two high-concentration formulations, Adalloce Prefilled Syringe Inj 40mg/0.4mL and Adalloce Pen 40mg/0.4mL, to the domestic market.
Currently, the domestic adalimumab market is dominated by prescriptions for high-dose formulations. Patients prefer high-concentration products as they can be administered in a single injection and with less frequency.
Samsung Bioepis, which first entered the domestic Humira biosimilar market in July 2020, received approval for Adalloce's high-concentration formulation in January 2023 and has been supplying it through Yuhan Corp. since June.
LG Chem received approval for two Humira biosimilars from the Ministry of Food and Drug Safety in December last year, a year after applying.
The approved products are Xelenka Prefilled Syringe Inj 20mg/0.2mL, 40mg/0.4mL, and 80mg/0.8mL and Xelenka Autoinjector Inj 40mg/0.4mL in two doses. It is the third company to enter the domestic Humira biosimilar market after Samsung Bioepis and Celltrion.
"We plan to launch Xelenka in the second half of 2024," an LG Chem official said in response to Korea Biomedical Review’s question on when and how it would market the product, including joint sales with domestic partners. "We are still considering sales strategies."
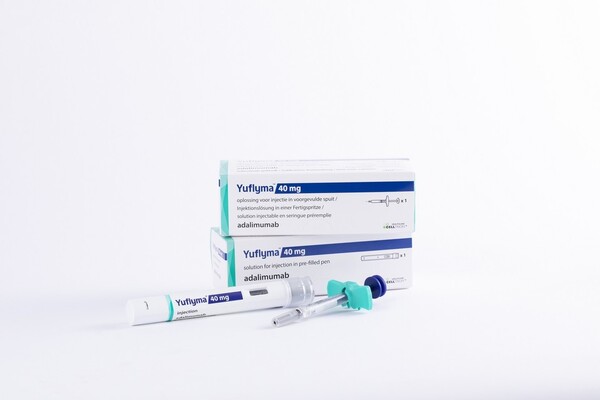
Celltrion entered the adalimumab market in October 2021 by winning the approval of Yuflyma Pen Inj 40mg/0.4mL and Yuflyma Prefilled Syringe Inj 40mg/0.4mL from the Ministry of Food and Drug Safety. In June 2022, the company received approval for high-dose and high-concentration products, Yuflyma Pen Inj 80mg/0.8mL and Yuflyma Prefilled Syringe Inj 80mg/0.8mL.
Celltrion changed the production site of Yuflyma from its overseas plants to domestic ones. In November 2022, Celltrion received approval for an additional high-concentration product named Celltrion Yuflyma Pen Inj 40mg/0.4mL.
Abby's Humira treats autoimmune diseases, including rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, and plaque psoriasis.
It is a blockbuster drug with global sales of $21.2 billion (27.92 trillion won) in 2022. The domestic market for adalimumab is valued at about 100 billion won.
Related articles
- Celltrion seeks US nod for interchangeability between Yuflyma and Humira
- LG Chem's Humira biosimilar for autoimmune disease treatment gets nod in Korea
- Korean biotech firms spearhead biosimilar race for MSD's Keytruda as patent expiry looms
- Samsung Bioepis begins US approval process for Humira biosimilar’s interchangeability
- Celltrion launches high-dose version of Yuflyma in US
- Korean regulator OKs Samsung Bioepis’ Soliris biosimilar
- Psoriasis patients should manage obesity to improve drug response
- Samsung Bioepis, Biogen lose Humira biosimilar patent fight in Germany

