The Ministry of Food and Drug Safety has granted approval to two more digital therapeutic (DTx) devices, developed by Nunaps and Share&Service. These devices, which are the third and fourth authorized DTx in the country, utilize software to assist in cognitive improvement and respiratory rehabilitation.

DTx devices are defined as software medical devices that intervene therapeutically based on scientific evidence to prevent, manage, or treat medical impairments or diseases. These tools are being increasingly utilized to improve patient outcomes in various medical settings.
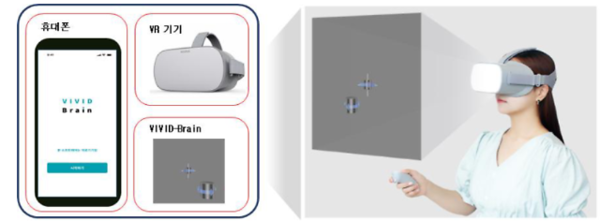
Nunaps received approval for VIVID Brain, a mobile application designed for patients with vision impairments due to brain disorders.
The application provides a 12-week visuoperceptual training program aimed at expanding the visual field of its users. This tool addresses the specific needs of patients with visual limitations from neurological conditions.
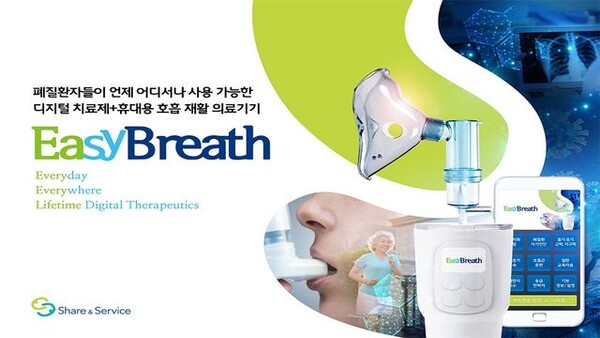
The ministry also approved Share & Service's EasyBreath, which offers an eight-week customized respiratory rehabilitation program for individuals with chronic obstructive pulmonary disease (COPD), asthma, and lung cancer.
This application focuses on enhancing aerobic capacity and reducing breathlessness, thereby improving users' quality of life.
Notably, the ministry supported these products from their development stages through to the clinical trial designs, conducting thorough and scientific evaluations.
Additionally, both devices have been designated as innovative medical devices, a status that expedites their integration into medical practice.
"This clearance demonstrates that digital therapeutic devices can be used not only to treat disease but also to alleviate disability," Minister of Food and Drug Safety Oh Yoo-kyung said. "We believe that digital therapeutic devices can help improve the quality of life for patients suffering from diseases and disabilities."
The ministry will continue to make domestic regulations a global standard so that domestic companies can continue to develop new innovative products that lead the global market, Oh added.
Oh also stressed that the ministry build a solid foundation for regulatory support, such as close consultation with regulatory experts and preemptive standardization, to accelerate product launches and enable digital therapeutic devices to be utilized for various diseases.

