OliX Pharmaceuticals’ short interfering ribonucleic acid (siRNA)-based hypertrophic scar treatment OLX10010 (OLX101A) has raised questions in the market due to the omission of certain information, including safety assessments, in the published results of its phase 2a clinical trial.
On Wednesday, OliX announced publicly that it received the U.S. phase 2a clinical study report (CSR) for OLX10010 from a U.S. contract research organization (CRO).
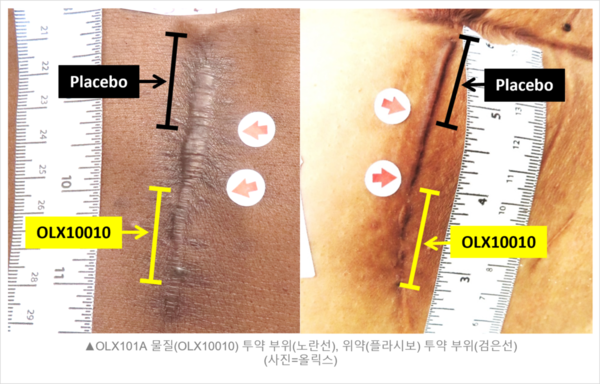
The study evaluated the efficacy of OLX10010 as an adjunctive therapy for recurrence control following scar reconstruction in U.S. patients who underwent scar reconstruction for two years, from August 2021 to July 2023, following the U.S. Food and Drug Administration (FDA) approval in 2020.
Planned for proof-of-concept (PoC), the phase 2a study was designed as a randomized, double-blind, within-individual comparison, placebo-controlled study. While the number of patients enrolled in this disclosure is 20, the actual number of patients enrolled totaled 22, according to the Clinical Trials Registry, a clinical trial information website of the National Institutes of Health (NIH).
"Observations over 48 weeks post-dose confirmed that OLX10010 maintained similar levels of inhibition of scar recurrence as seen in the topline results," OliX said in a news release regarding the receipt of the phase 2a results.
"This is the first formal proof-of-concept in humans for our RNA interference drug technology," OliX Pharmaceuticals CEO Lee Dong-ki said. “We plan to use the CSR to establish global partnerships and discuss late-stage clinical development plans.”
However, the disclosure only included POSAS (patient and observer scar assessment scale) scores at 24 weeks post-scar reconstruction, just like the topline data released last April. However, the market expected to see safety results from a safety assessment at week 48, which was not included in the disclosure.
The previously disclosed efficacy results also do not provide a direct statistical comparison of the effectiveness of the treatment and control groups. The change in mean POSAS scores within each group was significant, making it difficult to determine whether OLX10010 was actually superior to placebo based on the data presented.
Industry insiders are interested in whether OliX will release a more detailed analysis of the trial data or a full trial report in the future. On the day of the OLX10010 phase 2a results announcement, Olix's stock price closed at 15,310 won ($11.1), down slightly from the previous day's close of 15,570 won.
Meanwhile, Hugel, which had been developing hypertrophic scar treatment OLX10010 in Korea with OliX for 10 years since 2013, announced in May last year that it terminated the technology transfer agreement for OLX10010 (Hugel's development name BMT101) and the domestic phase 2a study earlier than scheduled. Hugel disclosed that the decision was based on a change in internal business policy.
Related articles
- OliX Pharmaceuticals unveils preclinical data for hair loss treatment at World Congress for Hair Research
- OliX Pharmaceuticals shares plunge 17% following failed licensing agreement
- Olix proves hair loss drug’s safety, tolerability in Australian P1 study
- OliX strikes $630 million deal with Lilly for obesity and liver disease drug
- OliX books $3 mil. payout from Hansoh as liver-targeted RNA drug moves forward in China

