Hanmi Pharm said Friday it presented clinical results of its low-dose three-drug combination for the treatment of hypertension at the annual meeting of the European Society of Hypertension (ESH 2024), held from May 31 to June 4 in Berlin, Germany.
The phase 3 clinical trial study, HM-APOLLO-301, compared changes in systolic and diastolic blood pressure after eight weeks of treatment with either a low-dose triple combination or a standard-dose single agent in adult patients with mild to moderate hypertension.
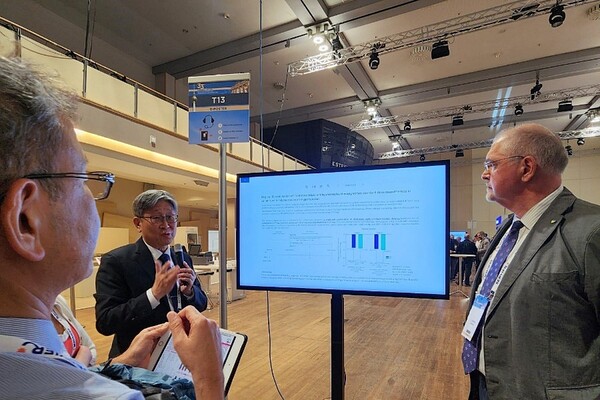
The results showed that after eight weeks of treatment, systolic blood pressure in the low-dose triple combination arm decreased by approximately 18.3 mm Hg from baseline, while that in the standard-dose single-agent arm decreased by approximately 19.4 mm Hg. This indicated that the treatment effects were similar between the two arms. There was no significant difference in the incidence of adverse events, confirming that the efficacy and tolerability of the low-dose triple combination was similar to the standard-dose monotherapy, according to Hanmi Pharm.
The study drew attention from academics as it reflects the next-generation treatment concept that a low-dose combination of three antihypertensive components may be effective as an initial treatment regimen for hypertension.
"Hypertension, a representative chronic disease, has not yet been clearly defined in terms of its pathogenesis," said Dr. Rhee Moo-yong, professor of cardiology at Dongguk University College of Medicine, in a presentation of the results. "Breaking away from the clinical inertia of starting treatment with monotherapy, a new approach that simultaneously blocks multiple pathological pathways will allow us to more effectively improve the prognosis of patients."
The findings are significant because they confirm both the efficacy and safety of a low-dose, three-drug combination as an initial treatment for patients with mild to moderate hypertension, Rhee said.
"This may broaden the options for initial treatment of hypertension and improve patient compliance."
Related articles
- Hanmi Pharm's Rolontis moves quickly to expand overseas markets
- GC Biopharma, Hanmi Pharm's Fabry disease drug gets US FDA orphan drug status
- Hanmi Pharm's efocipegtrutide phase 2 trial for MASH gets green light from IDMC
- Hanmi Pharm partners with MSD for clinical trial of cancer therapy BH3120
- Hanmi Pharm's novel drug receives INN name 'efpegerglucagon' for treating congenital hyperinsulinemia
- Smart ring for 24/7 blood pressure monitoring costs less than $5 for Korean patients
- Hanmi Group's family dispute may have ended
- Hanmi Pharm, MEDiC Life Sciences to co-develop new biomarkers for predicting the efficacy of cancer drugs

