NeuroLogica Corp., a U.S. subsidiary of Samsung Electronics, received additional U.S. FDA clearance on July 10 for new features of OmniTom Elite Photon Counting Detector (PCD), an advanced CT scanner. The new features include an ultra-high resolution mode, PCD-specific applications, expanded scanning range, and continuous helical scanning capabilities.
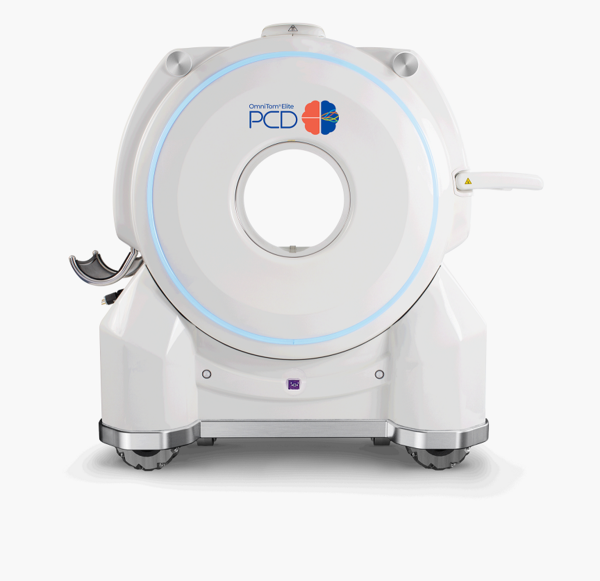
The OmniTom Elite's PCD technology is a next-generation CT scanning advancement that uses semiconductors to deliver higher resolution and lower electronic noise in CT images compared to conventional scintillator-based methods.
Samsung Medison, a Korean medical device maker uner Samsung Electronics, said that the FDA-cleared upgrade to the OmniTom Elite PCD introduces an ultra-high resolution mode for superior image quality. The imaging can also be adjusted to remove residual contrast seen in CT scans taken after contrast has been administered.
The OmniTom Elite PCD was the world's first mobile CT with PCD technology to receive FDA clearance in 2022. “With these new features, the OmniTom Elite PCD is poised to be even more competitive in the global mobile CT market,” Samsung Medison said.
“We are pleased to receive additional FDA clearance for the development of new features to improve diagnostic convenience,” said Yoo Kyu-tae, GM of Samsung Electronics HME Business and CEO of Samsung Medison. “We will continue to lead the development of new technologies in the medical imaging field to improve diagnostic accuracy while providing convenience to medical staff.
In Korea, the Ministry of Food and Drug Safety approved OmniTom Elite PCD last year, following its existing FDA approval.
Meanwhile, the OmniTom Elite PCD is pending to receive CE MDR (European Union Medical Device Regulation) certification for its new FDA-approved features.

