Recent studies have revealed that TLR agonists, which activate toll-like receptors (TLRs) crucial for immune responses against infections, can stimulate the immune system to target and eliminate cancer cells.
Although preclinical studies had suggested that CVI-CT-001, an immuno-oncology drug candidate developed by the CHA Vaccine Research Institute using the TLR-targeting adjuvant L-pampo, could kill specific cancer cells and transform the tumor microenvironment into a highly immunogenic state, the exact mechanisms remained unclear—until now.

The CHA Vaccine Research Institute and the MOGAM Institute for Biomedical Research said Friday that their joint research, published in the journal Scientific Reports in July, used artificial intelligence (AI) and bioinformatics to uncover the mechanisms behind CVI-CT-001’s cancer-killing effects.
In this collaborative research, CHA Vaccine Research Institute handled experimental design and data production, while MOGAM Institute analyzed the data using AI and bioinformatics technologies. The study employed RNA sequencing (RNA-seq) and advanced computational methods to investigate how the drug induces cancer cell death.
The study analyzed transcriptome data from cell lines treated with CVI-CT-001, revealing that the drug activates TLR signaling pathways, particularly in prostate and colon cancer cells.
This activation leads to oxidative phosphorylation (OXPHOS) and the production of reactive oxygen species (ROS) via the PI3K-AKT and JAK-STAT pathways, selectively inducing cancer cell death.
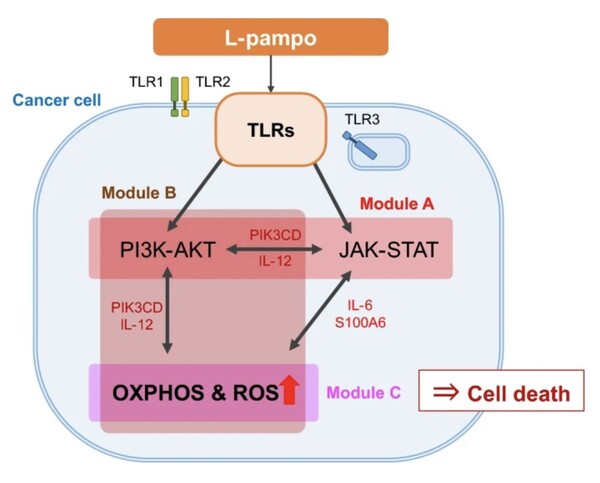
The study also identified specific gene clusters associated with this process, suggesting that CVI-CT-001’s effectiveness may stem from its ability to enhance the sensitivity of prostate cancer cells to treatment.
Researchers built a gene co-expression network from three cell lines—human monocytes, prostate cancer, and colon cancer cells—uncovering gene expression differences across these lines. Leveraging AI and bioinformatics tools from the MOGAM Institute, the team reconstructed subnetworks of key molecular signals and identified genes likely to play critical roles in CVI-CT-001’s cancer cell-killing process.
“This research has captured subtle drug response signals within RNA-seq data using AI algorithms,” said Shin Hyun-jin, president of the MOGAM Institute. “These findings suggest that AI technology can accelerate drug development by helping us understand the mechanisms of drug action.”
Yeom Jeong-seon, CEO of CHA Vaccine Research Institute, added, “Moving forward, we will continue to leverage AI technology to quickly assess the efficacy and mechanisms of new drug candidates.”
The full study detailing these findings is published in Scientific Reports.
Related articles
- ST Pharm and CHA Vaccine Institute agree to co-develop RNA-based immunotherapies
- CHA Vaccine Institute says its hepatitis B vaccine more effective than conventional vaccines
- CHA Vaccine Institute develops mRNA delivery system, addressing LNP-related limitations
- Mogam institute, SNUH to develop AI-powered rare disease knowledge base
- CHA Vaccine Institute, SML Biopharm collaborate for mRNA-based vaccines, therapeutics

