As millions worldwide grapple with incurable brain diseases that resist conventional drug treatments, researchers are exploring technologies that offer a more precise alternative. One such innovation is transcranial-focused ultrasound neurostimulation, which has shown promise in targeting damaged brain tissues.
However, its effectiveness has been inconsistent, as fixed stimulation parameters often fail to account for the unique complexity of each patient's neural architecture, sometimes exacerbating symptoms rather than alleviating them.
In a bid to outwit neural unpredictability, a research team led by Dr. Son Dong-hee from the Center for Neuroscience Imaging Research at the Institute for Basic Science (IBS) and Dr. Shin Mi-kyung from the biomedical engineering department at Sungkyunkwan University have developed a closed-loop nerve stimulation system that adapts to real-time changes in brain wave activity induced by ultrasound, offering a more personalized approach to treatment.
Their innovation, published in the journal Nature Electronics on Sept. 11, features an elastic electronic patch that adheres securely to the brain, introducing what the researchers term "patient-specific brain disorder control electronic medicine technology."
Traditional electrode devices, with their rigid and inflexible designs, struggle to conform to the brain's intricate and curved surfaces. As the brain shifts with micromovements, these devices lose their secure fit, leading to unreliable EEG measurements over extended periods. Additionally, the intense noise generated by negative pressure oscillations during ultrasound stimulation complicates the task further, rendering electrical EEG readings ineffective as real-time feedback.
These challenges disrupt neurosignal feedback from brain-integrated electrode devices, as sonication-induced oscillations create electrical artifacts that block optimal closed-loop transcranial-focused ultrasound neurostimulation. “Soft, brain-interfacing materials with robust tissue adhesion and conformability even on a curved surface are thus required” for creating neural devices that remain artifact-resistant during ultrasound stimulation, the researchers said.
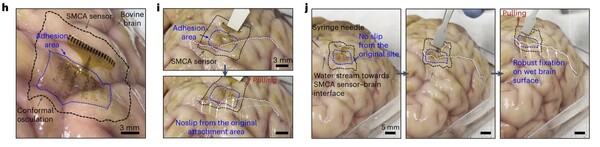
In response, Dr. Son and his team have developed a shape-morphing, cortex-adhesive (SMCA) stretchable electrode patch that adheres seamlessly to the brain’s surface, providing precise and consistent EEG measurements by adapting to the brain’s contours. Composed of a viscoelastic hydrogel adhesive, a stretchable 16-channel microelectrode array, and a self-healing polymeric substrate, the patch integrates with a pulse-controlled transcranial-focused ultrasound device.
Unlike traditional methods that are disrupted by the noise of ultrasonic nerve stimulation, this technology uses brain wave measurement technology to capture electrical signals directly from the brain’s surface, offering immediate feedback while diagnosing pathological brain waves without interference from ultrasound stimulation.

When the researchers applied their electronic patch to cerebral tissue, the adhesive hydrogel quickly absorbed fluid from the contact surface, swelling and adhering to the brain within seconds. The patch’s shape-shifting material, with viscoelastic properties, adapted easily to changes in shape, while its thermoplastic qualities allow it to soften at higher temperatures. “These features enable the patch to conform to the brain’s irregular cortical structures, adhering securely without gaps as it responds to the body’s heat,” the researchers said.
Once bonded to the brain's surface, the patch remains stable even under negative pressure oscillations, suppressing noise and enabling high-quality measurements of cortical conduction.
Equipped with a ‘patient-customized brain disease control electronic medicine system,’ the patch can detect brain waves with remarkable clarity, even during intense ultrasonic nerve stimulation. It also calibrates treatment parameters in real-time, delivering a precision-tuned therapeutic strategy that leverages immediate feedback from the brain’s electrical signals.
“For the first time, we were able to measure an individual patient's neural activity in response to ultrasonic stimulation in real-time, bringing us a step closer to personalized brain disease treatment,” said Dr. Son, adding that this technology is “expected to become a core technology for next-generation electronic medicine,” enabling precise diagnosis and personalized treatment of intractable neurological diseases.
In a rat model of induced epilepsy, this electronic patch maintained stable EEG monitoring even in freely moving animals. It precisely detected the pathological high-frequency signals that precede seizures, predicting the onset of full-blown seizures within minutes and triggering ultrasound stimulation.
"The stable neuro signaling performance allows our platform to function as both a chronic seizure predictor and an automatic trigger for neurostimulation by detecting epileptic high-frequency oscillations (HFO) with high accuracy," the researchers noted.
The full study detailing these findings is published online in Nature Electronics (Impact Factor: 33.7).

