Kuhnil Pharmaceutical has launched Slenyto SR Tablet, a melatonin-based insomnia treatment for children and adolescents.
As there has been no officially licensed insomnia treatment for children and adolescents in Korea, industry insiders are paying attention to the impact of Slenyto on the clinical field.
Developed by Israel-based Neurim Pharmaceuticals, Sleynto was launched on Thursday last week. It was approved for treating insomnia in children and adolescents aged two to 18 years with Autism Spectrum Disorders (ASD) and Smith-Magenis syndrome (SMS), whose symptoms have not improved with improved sleep hygiene.
“In other words, it has proved to treat pediatric insomnia through clinical trials,” a Kuhnil Pharm official said during a recent meeting with journalists covering the Korea Pharmaceutical and Bio-Pharma Manufacturers Association to explain the background and implications of the launch.
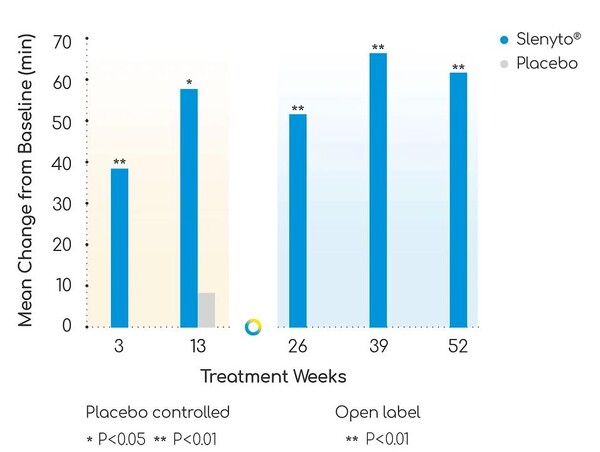
In the phase 3 trial, Slenyto increased sleep duration by 40 minutes at week 3 compared to placebo, with an average increase in total sleep duration of 62.08 minutes over four measurements at weeks 13, 26, 39, and 52. After dose optimization, sleep maintenance increased by 89.1 minutes at week 52. On the other hand, sleep latency, the time spent preparing for sleep but not falling asleep, decreased by an average of 48.6 minutes at week 52.
The study, conducted at 24 sites in Europe and the U.S., involved 125 children and adolescents aged two to 17.5 with ASD and neurological disorders who were divided into two groups—the Slenyto (60) and placebo (65). It measured sleep onset, sleep duration, and total sleep time after 13 to 52 weeks of Slenyto treatment. Of these children, 28.5 percent had comorbid ADHD, and 12.8 percent had comorbid epilepsy.
Interestingly, raising sleep quality in children also improved the quality of life of their caregivers.
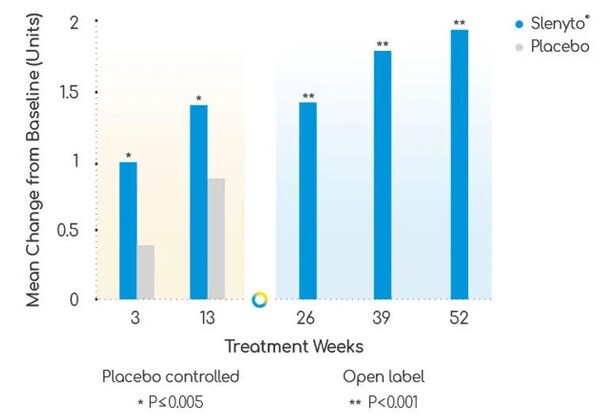
In the study, parents in the Slenyto group reported an average increase of two points in child sleep satisfaction on a scale of one to five, and nearly 50 percent of caregivers reported a 10 percent or greater increase in quality-of-life scores compared to pre-treatment.
There were no significant adverse events, which is the most common concern when using medications in children. In a two-year follow-up study, adherence to the tablets was close to 100 percent over the 24-month study period despite mild drowsiness. There were no adverse events, such as dependence, abuse, tolerance, or withdrawal after discontinuation.
“We have already confirmed melatonin's short- and long-term safety with Circadin (the same ingredient as Slenyto),” said Choi Seok-hong, head of Marketing 1 Division at Kuhnil Pharm. “The drug has confirmed it is a treatment for insomnia without tolerance or dependence.”
Choi noted that relevant guidelines in Europe and other countries require pediatric patients with SAD and SMS to be given melatonin first when administering medication, proving its safety.
He explained the effectiveness of Slenyto in detail.
“Many ASD patients have sleep problems due to a genetic disruption in the production of serotonin, leading to a decrease in melatonin that helps with sleep. In this case, many studies have confirmed that symptoms, including nighttime seizures and mood instability, lead to sleep deprivation and poor quality of life for caregivers of people with ASD,” Choi said. “Moreover, chronic insomnia reduces REM sleep necessary for restorative sleep processes, and leads to cognitive decline, increased aggression, hyperactivity, and fear in daily life, creating a vicious cycle.”
“In Europe, up to 75 percent of children with ASD are non-compliant with caregivers' instructions, so medication is often the only option, but conventional supplements (such as melatonin) are often short-acting and do not support long sleep patterns. Add to that the cognitive deficits and obsessive-compulsive symptoms of children with ASD. It's very difficult for them to make changes to their daily routines, such as eating and sleeping, or to add a 'take a big pill' routine.”

In contrast, the extended-release tablet form of Slenyto is taken 30 minutes before bedtime to release melatonin, which supports restful sleep by mimicking normal sleep patterns for up to seven to eight hours. In addition, the nearly spherical shape and small three-millimeter tablet size make it easy to swallow. The tablets can be mixed with yogurt, juice, or ice cream, making them easy for children with ASD to take.
“Slenyto is the first melatonin formulation that has been shown to improve all three measures of sleep quality -- total sleep duration, time to fall asleep, and sleep maintenance,” Choi said. “We are confident that Slenyto will improve the quality of life for patients and caregivers by improving sleep quality and externalizing behaviors in children with ASD.”
Slenyto is available in 1mg and 5mg formulations, priced at 1,600 won ($1.2) and 4,000 won without coverage.
Related articles
- World Alzheimer's Day: One in 10 Koreans aged 65 and older has dementia
- The impact of stigma and service gaps on mental health in Korea vs US
- Epilepsy rises in older adults, leading to 'epileptic sudden death’
- A hidden reason for older adults’ insomnia: craving for a good night's sleep
- How a culture of sleepless success in Korea impacts health and society
- The greater the grit, the better the sleep: study
- Asleep’s AI-powered sleep apnea screening app now prescribed at SNUBH
- Narcotic ADHD drug prescription jumped over 2.5 times in 5 years
- Kuhnil Pharm appoints former Takeda Korea head Moon Hee-seok as co-CEO

