Jeil Pharmaceutical announced on Monday the launch of Jaqbo (zastaprazan citrate), a new potassium-competitive acid blocker (P-CAB) that will enter Korea’s peptic ulcer drug market, valued at 1.3 trillion won ($1 billion). This is the first time in Jeil Pharmaceutical's 65-year history that the company has launched a new drug through in-house development.
Jaqbo, developed by Onconic Therapeutics, a subsidiary of Jeil Pharmaceutical, received approval in April as Korea's 37th new drug. It will be covered by health insurance for the treatment of erosive gastroesophageal reflux disease (GERD) starting Oct. 1, with an insurance price set at 911 won per 20 mg tablet, according to the Ministry of Health and Welfare.
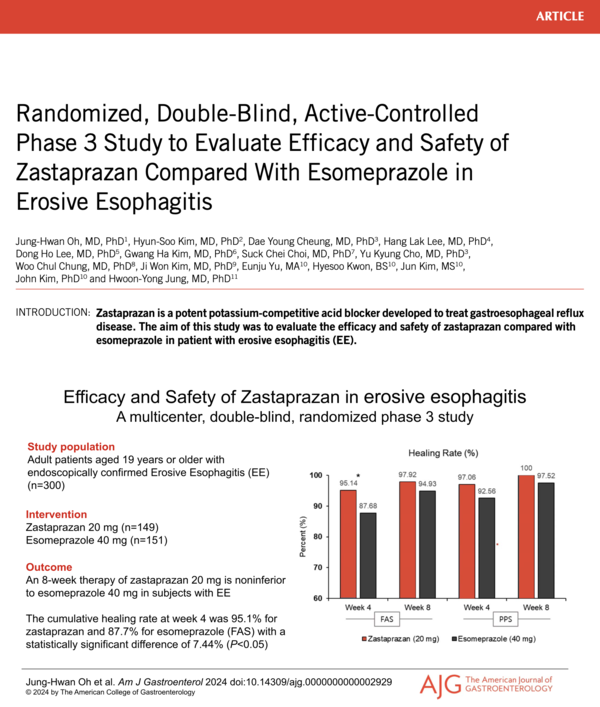
The drug is recognized for its rapid onset of action and extended duration, making it an innovative alternative to traditional proton pump inhibitors (PPIs), which have been the standard treatment for stomach acid-related conditions for the past three decades. Phase 3 clinical trial results for Jaqbo were published in August in the American Journal of Gastroenterology, highlighting its efficacy and safety in patients with erosive gastroesophageal reflux disease (GERD) and comparing the new P-CAB drug to esomeprazole, a commonly used proton pump inhibitor (PPI).
While PPIs have effectively managed stomach acid issues, they are not without limitations. Challenges such as delayed action, genetic variations affecting metabolism (specifically CYP2C19 polymorphism), and the occurrence of nocturnal acid breakthrough have led to patient dissatisfaction. Additionally, PPIs often require administration on an empty stomach, complicating patient adherence.
In contrast, Jaqbo operates through a unique mechanism that competitively inhibits stomach acid secretion by disrupting the binding of potassium ions to the proton pump. This characteristic allows Jaqbo to maintain stability in the acidic environment of the stomach, enabling immediate effectiveness without the need for activation by stomach acid. “This unique property of P-CAB allows it to bind to the proton pump regardless of stomach acid levels,” said a representative from Jeil Pharmaceutical.
As a result, while traditional PPIs can take four to five days to reach maximum effectiveness, Jaqbo works immediately after ingestion. Its long half-life further enhances its ability to treat nighttime heartburn through prolonged suppression of stomach acid. Additionally, since Jaqbo does not require activation by acid, it can be taken regardless of meal times, improving convenience for patients.
Since its launch, Jaqbo has garnered attention as a potential new treatment option in the domestic peptic ulcer market. Prior to its market entry, Onconic Therapeutics selected Jeil Pharmaceutical and Dong-A ST as partners for the domestic sales and marketing of Jaqbo, signing a joint sales agreement on Sept. 5. This collaboration allows Jeil Pharmaceutical and Dong-A ST, two prominent players in the domestic digestive health market, to jointly promote Jaqbo across all hospitals in Korea.
Following a plan of action meeting in August that included over 400 sales and marketing executives, Jeil Pharmaceutical has initiated a series of launch symposiums in major cities, starting with Seoul last Tuesday and continuing in Daegu, Daejeon, Busan, and Gwangju. Jeil Pharmaceutical said these events mark the beginning of comprehensive marketing efforts following the product launch.
To support the rollout, the company will provide patients and healthcare professionals with detailed product information and the latest academic research through various channels, including symposiums, regional academic gatherings, and major gastroenterology conferences.

According to Jeil Pharmaceutical, Jaqbo has already received recognition for its technology and product quality in international markets. Last year, the company signed a $127.5 million technology export contract with the Chinese pharmaceutical firm Livzon Pharmaceutical Group for development and commercialization. In May, Jeil also entered into a technology transfer agreement with a global pharmaceutical company in India, which the company declined to name for confidentiality reasons.
Earlier this month, it also secured technology export agreements with 19 countries in Latin America, including Mexico, Argentina, Chile, and Colombia, bringing the total to 21 countries worldwide. A Jeil Pharmaceutical representative said the company is “actively pursuing additional technology export contracts," positioning Jaqbo as a "potential global blockbuster drug.”
Currently, clinical trials are underway to expand Jaqbo's indications and formulations. In addition to its approval for erosive GERD, trials are exploring its use in preventing gastric ulcers and NSAID-induced peptic ulcers, as well as the development of new formulations such as oral disintegrating tablets.
According to pharmaceutical market research firm UBIST, the domestic peptic ulcer drug market has shown consistent growth, rising from 801 billion won in 2019 to 1.266 trillion won in 2023.
In the first quarter of 2024, PPIs held a 53.7 percent market share, while P-CABs accounted for 19.5 percent. In the second quarter, PPIs maintained a 53.4 percent share, with P-CABs increasing to 20.2 percent. Given that P-CAB formulations have only been in the peptic ulcer market for four years, Jeil Pharmaceutical anticipates the pace of market share expansion to accelerate.
“Jaqbo is a valuable product that our company has been developing for a long time, using significant human and material resources through Onconic Therapeutics,” said a Jeil Pharmaceutical representative. “We expect the share of P-CAB preparations in the peptic ulcer market to grow, and with the attention Jaqbo has received since its launch, we believe it will quickly establish itself and increase its influence as a new treatment option.”
Related articles
- Onconic Therapeutics to launch Jaqbo in 19 Latin American countries
- Onconic Therapeutics partners with Jeil Pharm, Dong-A ST for Jaqbo sale
- Green light is on for Jaqbo’s reimbursement to speed up P-CAB drugs’ growth
- Onconic Therapeutics passes preliminary Kosdaq IPO review
- Jeil Pharm's Jaqbo falls short in Korea’s booming P-CAB market with weak 1st-month sales
- Onconic Therapeutics to showcase drug pipeline at JP Morgan Healthcare conference 2025
- Jeil Pharm scores nod for multi-drug resistant gram-negative bacterial infection treatment in Korea
- New GERD drug Jaqbo wins grand prize in the Korean New Drug Development Award

