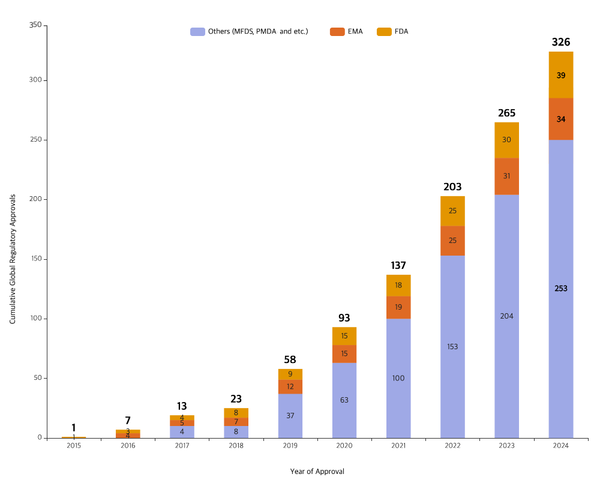
Samsung Biologics, the biotech unit of Korea's Samsung Group, said Thursday that it has received 326 global regulatory manufacturing approvals just 13 years after its founding.

Regulatory manufacturing approval confirms that a company’s biopharmaceutical manufacturing processes meet good manufacturing practice (GMP) standards, ensuring compliance with quality requirements. For contract manufacturing organizations (CMOs) like Samsung Biologics, such approvals are necessary to market products globally.
“Securing orders from global pharmaceutical companies and rapidly obtaining regulatory certifications have fueled our unprecedented growth in the CDMO sector,” said John Rim, CEO of Samsung Biologics.
As of September, Samsung Biologics had received 326 approvals, including 39 from the U.S. Food and Drug Administration (FDA) and 34 from the European Medicines Agency (EMA). The company attributed the growth in approvals to expanded production capacity and increased contract orders while maintaining a high pass rate for regulatory inspections.
Samsung Biologics highlighted workforce development, electronic data management systems, and remote inspection capabilities as key factors in successfully passing stringent regulatory inspections.
The company formed a specialized inspection team immediately after its establishment in 2011 to develop skilled professionals. The team focused on regulatory and client inspections, analyzed new guidelines, and trained staff to meet inspection standards. As a result, the number of personnel qualified to handle global manufacturing approvals has grown from around 70 in 2015 to approximately 550 today.
The company also introduced electronic document and quality management systems to digitize the extensive data generated during production, ensuring compliance with pharmaceutical manufacturing standards. According to Samsung Biologics, this digital approach enables real-time storage and access to data, allowing for quick responses to regulatory verification requests.
Samsung Biologics also enhanced its remote inspection capabilities by launching the Live-Virtual System, which uses multiple camera angles to create a virtual factory tour that resembles an on-site visit. An IT support team is available during inspections to resolve any technical issues, and according to Samsung Biologics, this system enabled the company to conduct over 184 remote inspections with regulatory agencies and clients during the Covid-19 pandemic.
"We will continue to make every effort to timely supply high-quality biopharmaceuticals to the market through continuous technological innovation based on quality management," said Rim.
Related articles
- Yuhan, Samsung Biologics among World's Most Trusted Healthcare Companies by Newsweek
- Samsung Biologics becomes 1st Korean CDMO to join US nonprofit group
- Samsung Biologics breaks record with over ₩2 trillion sales in H1
- Samsung Biologics announces record-breaking $1.06 billion CMO contract
- Samsung Biologics launches new high-concentration drug formulation platform
- Samsung Biologics signs record $1.24 billion CMO deal with Asian pharma
- Samsung Biologics achieves record revenue in Q3, driven by expanded product offerings
- Samsung Biologics secures over ₩5 trillion in annual CMO orders

