Boston Scientific Korea said its FARAPULSE Pulsed Field Ablation (PFA) system received approval from the Ministry of Food and Drug Safety (MFDS), marking the first medical device approved for pulsed field ablation (PFA) in Korea.
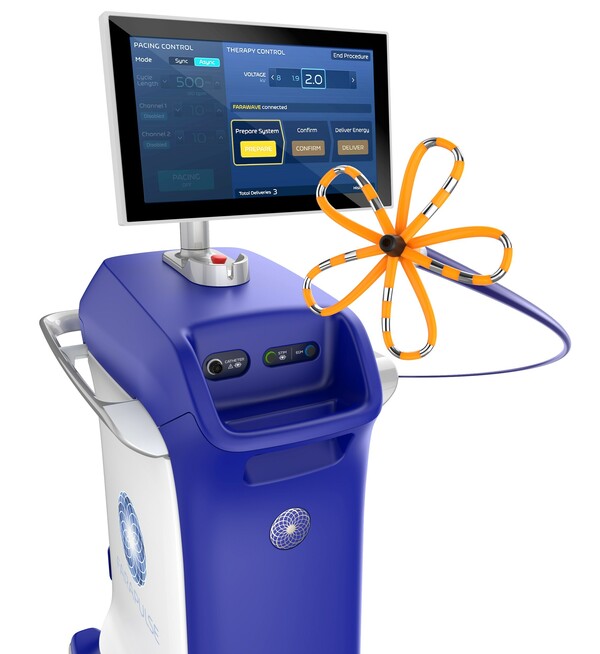
Unlike traditional thermal ablation techniques that rely on high or low temperatures, the FARAPULSE PFA System uses electrical fields to selectively target abnormal tissue, minimizing damage to surrounding structures like the esophagus. This novel approach is gaining traction as a promising alternative for atrial fibrillation (AF) treatment.
The FARAPULSE PFA System comprises the FARAWAVE PFA Catheter, the FARASTAR Pulsed Field Ablation Generator, and the FARADRIVE Steerable Sheath. The FARAWAVE catheter, notable for its flower and basket-shaped design, is particularly adjustable to accommodate a wide range of anatomical variations, enhancing ease of use and reducing operator variability during procedures.
Boston Scientific has demonstrated the system’s efficacy and safety through the ADVENT study.
The study, a randomized clinical trial comparing PFA to thermal ablation, found that PFA is not only as safe and effective as thermal methods but also shortens procedural time and improves the operator’s learning curve.
Additionally, data from the MANIFEST-17K registry, which analyzed real-world outcomes in over 17,000 patients treated with the FARAPULSE system, reported no instances of permanent nerve damage, pulmonary vein stenosis, or esophageal injury, further confirming its safety profile.
“We are thrilled to introduce the FARAPULSE PFA System, which has accumulated diverse clinical evidence worldwide and has been used in over 125,000 patients globally,” Boston Scientific Korea Managing Director Jung Ae-ri said. “We look forward to leading the domestic PFA market and improving treatment outcomes for atrial fibrillation patients in Korea.”
The FARAPULSE PFA system was designated as a Breakthrough Device by the U.S. FDA in 2019, received European CE Mark approval in 2021, and has since been approved in more than 65 countries, including the U.S., Japan, and across Europe. In Korea, the system is expected to launch following the Ministry of Health and Welfare’s New Medical Technology Assessment.
Related articles
- Boston Scientific launches intragastric appetite suppression balloon 'BIB System' with coverage
- Boston Scientific’s Lumenis Pulse 120H will support stone treatment in Korea
- Boston Scientific voluntarily recalls catheterizers
- Severance, ‘Korea’s 1st’ international training center for 3D pulsed field ablation
- Myongji Hospital successfully performs PFA for atrial fibrillation
- Boston Scientific’s TheraSphere reshapes liver cancer care in Korea as radioembolization eclipses chemo

