Lung cancer remains the number one cancer killer.
Epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) is especially prevalent in Asians, including Koreans, with a prevalence of about 50 percent.
Tagrisso (osimertinib) is the only full-cycle targeted anticancer drug used to treat EGFR-mutant NSCLC from early to late stages. It improves cure rates by reducing the risk of recurrence in early-stage patients, prolonging life, and improving quality of life in late-stage patients.
Professor Lee Se-hoon of the Department of Hematology-Oncology at Samsung Medical Center recently highlighted the clinical value of Tagrisso adjuvant therapy in treating patients with early-stage EGFR-mutant NSCLC on the YouTube channel “Medical Library” run by the Korean Doctors' Weekly, a sister paper of Korea Biomedical Review.
“Lung cancer has a significantly higher recurrence rate than other cancers, even if the patient has an early stage,” Professor Lee pointed out.
Lee explained that, in stage 1, the recurrence rate is about 20 percent; in stage 2, it is about 40 percent; and in stage 3, 60-70 percent of patients will eventually experience recurrence.
“To reduce the risk of recurrence in patients with early-stage lung cancer with such a high recurrence rate, we have been using adjuvant chemotherapy, but it only reduced the recurrence rate by about 4 percent,” Lee said, suggesting that there is a significant unmet need in this area.
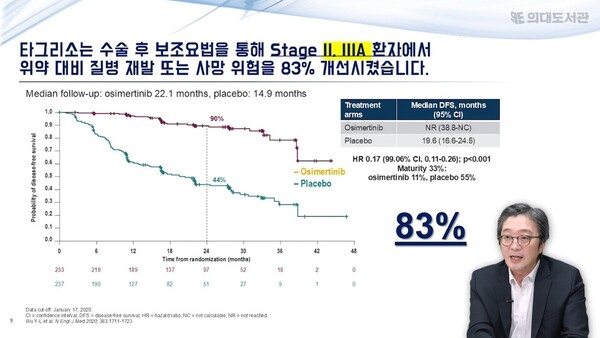
According to Lee, Tagrisso has successfully reduced recurrence rates by about 80 percent as a “post-surgical adjuvant” in treating EGFR-mutant NSCLC, which is particularly prevalent in Koreans.
The ADAURA study, Tagrisso's phase 3 trial, compared the effectiveness of Tagrisso and placebo after surgery in patients with stage 1B to stage 3A EGFR-mutant NSCLC. It found that Tagrisso adjuvant therapy improved the primary endpoint of disease-free survival (DFS) by 83 percent compared to placebo (in stage 2-3A patients).
Tagrisso adjuvant also improved DFS by 80 percent compared to placebo across all stages tested (stages 1B through 3A).
“For example, if you had 10 people relapse over five years, only two people relapsed (with Tagrisso adjuvant),” Lee said. “That's eight people who didn't relapse, which is tremendous data.”
Tagrisso adjuvant therapy also improved overall survival (OS) compared to placebo. It reduced the risk of death by 51 percent.
While many first- and second-generation EGFR-targeted agents have been studied as postoperative adjuvant therapy, Tagrisso is the only agent to demonstrate an improvement in DFS, followed by OS.
“The success of adjuvant Tagrisso in improving OS is remarkable,” Lee said, attributing the difference to Tagrisso's effects on the central nervous system (CNS).
In the ADAURA study, adjuvant Tagrisso reduced the risk of brain metastasis recurrence by 80 percent compared to placebo.
Lee said that in real-world clinical practice, the most important factor to consider for Tagrisso adjuvant therapy is staging. The more advanced the stage, the higher the risk of recurrence and the more effective Tagrisso.
Professor Lee added that Tagrisso adjuvant therapy can also be considered in patients at higher risk, depending on the pathology results of the resected cancer cells.
Related articles
- AstraZeneca's Tagrisso reaffirms its global standing in lung cancer treatment
- Korea's homegrown Leclaza gains momentum in NSCLC treatment, awaits global expansion
- Tepmetko offers potential follow-up cure for Tagrisso-resistant lung cancer patients
- New treatment for EGFR-mutant NSCLC: Tagrisso combined with chemotherapy approved
- New therapies lurbinectedin and tarlatamab bring hope for small cell lung cancer treatment
- Tagrisso expands indication for unresectable stage-3 EGFR-mutant lung cancer patients
- AstraZeneca showcases Tagrisso's expanding role in EGFR-mutant lung cancer at ELCC 2025

