Matica Biotechnology, the U.S. subsidiary of CHA Biotech, said on Tuesday it independently developed a high-resolution ion-exchange chromatography (IEX) method for analyzing the ratio of empty and full adeno-associated virus 8 (AAV8) viral vector capsids.

The presence of non-functional, or empty, viral vectors in final drug products is considered an impurity by the U.S. FDA, adversely affecting dosage accuracy and drug efficacy. This issue is recognized as one of the top challenges in cell and gene therapy (CGT).
The new technique utilizes ion exchange high-performance liquid chromatography (IEX-HPLC) to rapidly and accurately differentiate empty capsids from fully loaded ones. Published in Frontiers in Bioengineering and Biotechnology in October, the method reportedly achieves results more than five times as accurate as previous techniques.
Matica Bio’s approach aligns with standards set by regulatory agencies, including the International Conference on Harmonization (ICH) and the FDA. It provides analytical data within 30 minutes without the need for expensive equipment typically associated with conventional methods, such as analytical ultracentrifuges or mass spectrometers.
“One of the most surprising findings from the study was the remarkable resolution achieved between empty and full capsids, which often appear indistinguishable in many current analyses,” said a Matica Bio official. The separation of empty and full capsids demonstrated a resolution of 15 and maintained a linearity greater than 0.98, even across a broad range of viral particle concentrations (E+9 to E+13 vp/mL). “This marks a significant upgrade over other IEX capsid separation methods,” the official said.
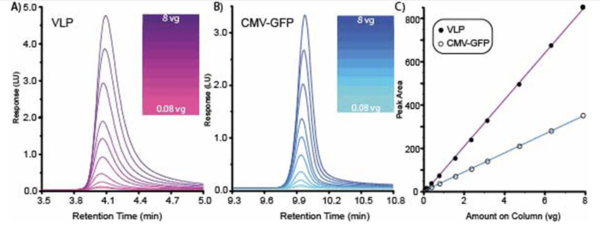
A greater distance between the peaks of full and empty capsids indicates clearer particle separation, enhancing test efficiency and yielding more precise quantitative results. Matica Bio achieved a resolution of at least 10, greatly exceeding the industry average, which typically ranges from 0.5 to 2.
“This innovative method allows us to conduct in-house analyses without the need for costly analytical ultracentrifugation (AUC) or mass spectrometry equipment, which can be burdensome for contract development and manufacturing organizations (CDMOs) and expensive for biotech companies,” said Daniel Mitchell, lead researcher of the study and head of analytical development and quality control at Matica Bio.
He noted that the company plans to extend the application of this method to various viruses, starting with AAVs and subsequently expanding to lentivirus and retrovirus.
As the production of viral vectors lacks full standardization, Matica Bio is also working on developing "real-time process analytical technology" and an "automation system" to enhance production timelines and facilitate the large-scale production of high-quality viral vectors.
Paul Kim, CEO of Matica Bio, emphasized that this new analytical method will enable the company to provide more efficient and reliable data analysis services to its clients.
Related articles
- Matica Biotechnology signs LOI with KaliVir Immunotherapeutics for CDMO partnership
- CHA Biotech's US subsidiary secures multiple viral vector CDMO contracts with biotech firms
- [Interview] Matica Biotechnology set to capitalize on CGT CDMO industry shifts, Biosecure Act opportunities
- Matica Biotechnology partners with Treovir on HSV vectors for pediatric brain tumor therapy
- CHA Biotech to appoint ex-Meritz advisor Choi as new CEO

