SINGAPORE -- By Kim Chan-hyuk/Korea Biomedical Review correspondent -- “The positive results from the JAVELIN Bladder 100 trial have been confirmed in real-world clinical practice, a significant finding for treating patients with advanced urothelial carcinoma in the Asia-Pacific region.”

Professor Jo Jeong-min of the Department of Hematology-Oncology at Ewha Womans University Mokdong Hospital said so during the genitourinary tumors research presentation session at the European Society for Medical Oncology Asia Annual Congress (ESMO Asia 2024) at the Suntec Convention & Exhibition Center in Singapore on Saturday (local time), presenting interim analysis results from the SPADE study.
The SPADE study is the first multicenter prospective observational study in the Asia-Pacific region to evaluate the real-world clinical effectiveness and safety of avelumab (Bavencio in trademark name) maintenance therapy after first-line platinum-based chemotherapy in patients with advanced urothelial cell carcinoma (UC).
Professor Jo took the podium with the “SPADE Study: Interim analysis of a prospective real-world study of first-line maintenance therapy with avelumab in patients with locally advanced or metastatic urothelial cell carcinoma in the Asia-Pacific region.”
“In the JAVELIN Bladder 100 phase 3 trial, avelumab maintenance significantly improved overall survival (23.8 months vs. 15 months) and progression-free survival (5.5 months vs. 2.1 months) compared with optimal adjuvant therapy,” Jo explained.
She added that the results were even better in the Asian subgroup analysis, with overall survival of 28.7 months vs. 18.2 months and progression-free survival of 5.6 months vs. 1.9 months.
The SPADE study enrolled 91 patients out of a planned 286 as of the data cutoff in April, with 67 percent (61 patients) on nivolumab maintenance therapy. The clinical characteristics of the patients included in the interim analysis showed a median age of 69 years (ranging from 48 to 92), and 83.3 percent were men.
By country, 83.3 percent (75 patients) were from Korea, followed by 13.3 percent (12 patients) from Taiwan, 2.2 percent (two patients) from Australia, and 1.1 percent (one patient) from India. The patient's ECOG (Eastern Cooperative Oncology Group) performance scale was 1 in 68.9 and 0 in 28.9 percent, while 80 percent showed normal renal function. Regarding disease status, 87.8 percent had visceral metastases, and 23.3 percent had bone metastases.
Regarding first-line chemotherapy, Professor Jo said, “Exactly 87.8 percent of patients received cisplatin-based chemotherapy, 7.8 percent received carboplatin-based therapy, and 4.4 percent switched between the two agents. Notably, 25.6 percent of patients received chemotherapy beyond the standard treatment duration of six cycles.”
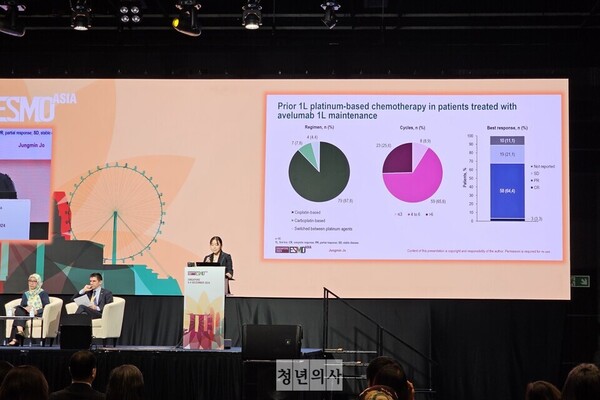
Concerning treatment outcomes, Jo said, “Some 64.4% of patients achieved a partial response (PR), 21.1 percent had stable disease (SD), and complete response (CR) was observed in 3.3 percent.”
The median duration of avelumab treatment was 98 days (it ranged from 14 to 352 days), and one patient died of disease progression during the study. In terms of reasons for discontinuation, 29 patients discontinued treatment: 22 due to disease progression, one due to adverse events, and six due to other reasons.
“Of the 29 patients who discontinued treatment, 27 (93.1 percent) received second-line chemotherapy, most commonly paclitaxel (seven patients) and enfortumab vedotin (six patients),” Professor Jo added.
“This is the first prospective real-world study in the Asia-Pacific region to confirm that the benefits of avelumab maintenance therapy seen in the JAVELIN Bladder 100 trial are consistent in real-world clinical practice,” Jo noted.
Asked about treatment selection criteria and considerations for prostate cancer patients, Professor Jo said, “It is very difficult to select the most appropriate drug for a patient because there are no biomarkers to predict treatment effectiveness. The patient selection criteria used in recent clinical trials are also difficult to apply in real-world practice.”
“For high-risk patients with liver metastases, we recommend combining pembrolizumab with chemotherapy, but for patients with a lower tumor burden, all three treatments can be considered.”
Related articles
- Merck Biopharma's Bavencio changes urothelial carcinoma treatment paradigm in Korea
- 'Bavencio improved progression-free survival in Korean urothelial carcinoma patients'
- ‘Bavencio becomes standard of care for urothelial cell cancer in 1 year of coverage’
- Padcev combo therapy shows excellent effect on Asian patients with urothelial carcinoma. What's next?

