A software medical device that utilizes artificial intelligence to screen for depression has gained approval for the first time in Korea.
The Ministry of Food and Drug Safety said it approved ACRYL-D01, an AI solution for diagnosing depression, last Friday. It is the first depression screening software licensed in Korea.
ACRYL-D01 is an AI-based depression probability indication software medical device (Class 2 item) developed by AI specialist Acryl.
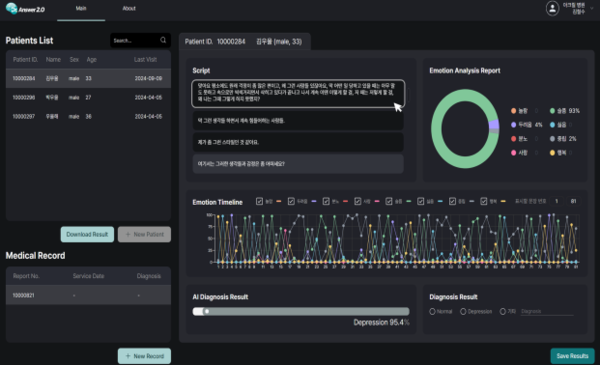
It aims to assist mental health clinicians in diagnosing depression by quantifying the probability of depression (0-100 percent) by digitizing the patient's interview notes according to the depression monitoring and evaluation criteria of the depression clinical practice guidelines.
Based on the interview transcripts of 2,796 patients in Korea, AI technology is used to analyze emotions and compare the diagnosis results of software medical devices and clinicians.
The patients' emotions (surprise, fear, anger, love, sadness, dislike, happiness, and neutral) are displayed in a circle graph, line graph, and probability. If the probability value is more than 50 percent, depression is displayed in the Diagnosis Result.
“Using the depression screening results predicted by this software, clinicians will be able to diagnose depression in patients with depressive disorders early and contribute to mental health management through continuous treatment,” the ministry said.
Related articles
- Starting next January, medical bills for depression diagnosis will be covered
- Pet loss syndrome can get serious enough to cause depression
- Migrant workers in Korea face isolation and rising suicide rates
- 'Childhood depression more common than ADHD as a cause of sudden academic decline'
- KAIST, University of Michigan develop wearable device to predict depression symptoms

