Lunsumio (mosunetuzumab), the first drug approved under the Global Innovative Products Fast Track (GIFT) program in Korea, was recently highlighted in long-term data showing excellent survival at three years with only eight cycles of fixed-duration treatment.
With the government having rejected many bispecific antibody therapies, citing the lack of long-term data, industry insiders are wondering if Lunsumio will be the first to break through the reimbursement bottleneck.
The February issue of Blood, an international journal from the American Society of Hematology, published nearly three years of follow-up data from Lunsumio's pivotal phase 1/2 study in patients with follicular lymphoma (FL).
The study evaluated the efficacy and safety of Lunsumio in second-line or higher therapy in 90 patients with relapsed or refractory follicular lymphoma, with a median follow-up of 37.4 months.
The study showed an investigator-assessed complete response (CR) rate of 60 percent and an objective response rate (ORR) of 77.8 percent, with a median duration of response (mDoR) of 35.9 months in the 70 patients who responded.
In addition, 49 of the 54 patients who achieved a complete response remained in complete remission at the end of treatment, with a median duration of remission not yet reached.
The Kaplan-Meier estimated 30-month remission rate was 72.4 percent, and the estimated three-year overall survival (OS) rate was 82.4 percent. Median overall survival has not yet been reached, and median progression-free survival was 24.0 months.
The median time to CD19+ B-cell recovery after eight cycles of Lunsumio was 18.4 months. No new cytokine release syndrome (CRS) or fatal, serious, or grade 3 or higher adverse events were reported.
“Mosunetuzumab fixed-duration administration demonstrates that durable remission can be achieved after three years of follow-up in patients with relapsed-refractory follicular lymphoma who have received multiple prior therapies,” researchers said. “At 37.4 months of follow-up, the complete response rate remained high at 60 percent, with most patients achieving durable benefit with eight cycles or less of treatment.”
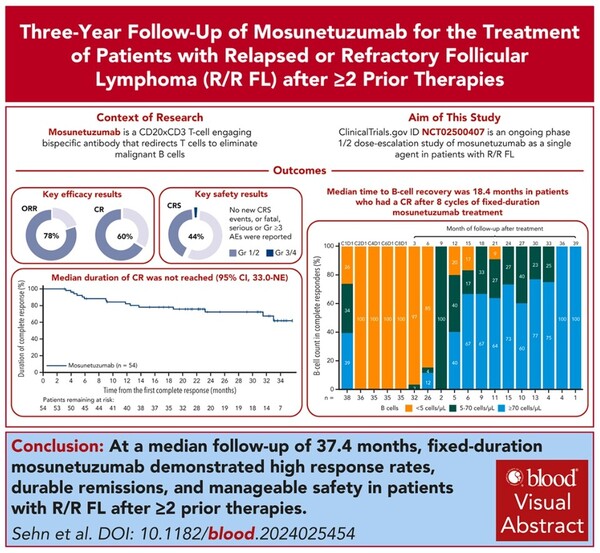
The researchers explained that this three-year update analysis is the longest follow-up report evaluating the efficacy and safety of bispecific antibody therapy in relapsed or refractory follicular lymphoma. The results of this analysis confirm and extend the primary analysis results, providing further evidence of clinically meaningful and durable benefits and a manageable safety profile of mosunetuzumab.
Recently, a number of applications for reimbursing bispecific antibody therapies in Korea have been submitted, but most of them have been stalled at the Cancer Disease Review Committee (CDRC) due to the lack of long-term data.
For example, Columvi (glofitamab) and Epkinly (epcoritamab) for diffuse large B-cell lymphoma (DLBCL) and Tecvayli (teclistamab) and Elrexfio (elranatamab) for multiple myeloma (MM) have failed to cross the threshold of the CDRC, the first gateway to insurance benefits.
However, Lunsumio is unique because it has demonstrated long-term survival benefits with a limited eight-cycle treatment.
Unlike conventional CAR-T therapies, it can be administered on an outpatient basis without hospitalization and has minimal pre-treatment with corticosteroids, which is a positive factor. It also has the potential to demonstrate cost-effectiveness due to its higher survival rate and longer duration of response compared to conventional anticancer drugs.
Above all, it has the longest duration of data compared to other bispecific antibody therapies, giving it an advantage in the reimbursement process.
As the first CD20-CD3 bispecific antibody and the first GIFT drug in Korea, industry insiders are wondering whether Lunsumio will be the first to secyre reimbursement approval.
Related articles
- Roche executives discuss innovation, collaboration in lymphoma treatment
- Roche Korea shares latest blood cancer treatment strategies using Columvi, Lunsumio
- Roche’s follicular lymphoma drug, 1st to receive approval through fast-track program in Korea
- [Column] CAR-T transformed treatment hopes for DLBCL, but Korea has a long way to go

