A research team at the Korea Advanced Institute of Science and Technology (KAIST) has identified a key mechanism that accelerates the replication of the SARS coronavirus, shedding light on how Covid-19 spreads so rapidly.
The team, led by Professor Lee Gwang-rog from the Department of Biological Sciences at KAIST, discovered that the nsp13 protein, a helicase essential for viral replication, possesses two distinct enzymatic activities that synergistically enhance the replication of SARS-CoV RNA.
Helicases are enzymes that function like a zipper, unwinding twisted structures of DNA or RNA to facilitate genetic replication and transcription. The KAIST research team found that nsp13 exhibits two critical activities -- helicase activity and RNA chaperone activity.
The helicase activity enables the enzyme to unwind double-stranded nucleic acids, such as DNA or RNA, into single strands, promoting viral replication and transcription. The RNA chaperone activity helps RNA molecules fold correctly, corrects misfolded RNA structures, and enhances RNA stability, thereby supporting cellular RNA metabolism.
For the coronavirus to rapidly spread, it must quickly replicate its genetic material and produce essential proteins. However, the precise reason behind the rapid replication of SARS-CoV RNA has remained unclear until now.
The KAIST research team revealed that nsp13’s helicase activity, combined with its newly discovered RNA chaperone function, accelerates the genetic replication process more efficiently than previously understood.
Nsp13 is highly conserved across different coronavirus variants, making it a crucial target for vaccine and antiviral drug development. However, a comprehensive understanding of its molecular mechanism was lacking.
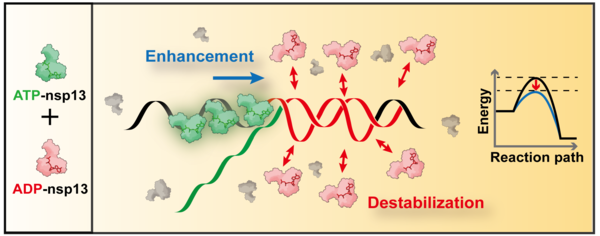
The study found that nsp13 hydrolyzes ATP (adenosine triphosphate), the primary energy source for enzymatic activities. When ATP is broken down, it releases chemical energy, which allows nsp13 to unwind the twisted RNA structure.
This process produces ADP (adenosine diphosphate) as a byproduct. Notably, the research team discovered that the newly generated ADP rebinds to nsp13, activating its RNA chaperone function. This additional function destabilizes secondary RNA structures, further accelerating the replication process.
Through this discovery, the KAIST team demonstrated that helicase activity and RNA chaperone activity operate in a synchronized manner to enhance viral RNA replication.
"This research uncovers a novel function of helicase enzymes, showing that ADP can activate RNA chaperone activity,” Professor Lee said. “Our findings expand the understanding of helicase functionality and provide a crucial breakthrough for developing effective therapies and vaccines against SARS coronavirus and its variants."
Nucleic Acids Research published the results of the study.
Related articles
- KAIST, University of Michigan develop wearable device to predict depression symptoms
- KAIST unveils new wearable robot for paraplegics, set to compete at Cybathlon 2024
- KAIST's unpowered medical oxygen generator picked as Top 20 Dyson Award
- New human coronavirus detected in infant with pneumonia, for 1st time in Korea
- KAIST researchers identify key factor behind immunotherapy resistance in lung cancer

