Coreline Soft has scored its 11th FDA clearance, winning 510(k) approval for AVIEW 2.0, the latest version of its AI-powered imaging platform.
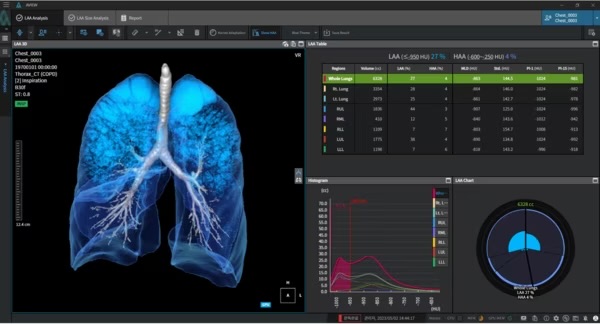
Coreline said Tuesday that AVIEW 2.0 includes upgrades in integration, security, and UI/UX to enhance data extraction and communication across its product lineup.
The platform supports several clinical applications, including AVIEW CAC for detecting coronary artery calcium linked to heart disease, AVIEW COPD for identifying chronic obstructive pulmonary disease, and AVIEW LCS for lung disease screening.
With FDA approval in hand, Coreline is focusing on expanding its partnership with U.S.-based 3DR Labs, aiming to provide additional AI software. The company is also accelerating its expansion into Europe and Japan, driven by the new certification.
Related articles
- Coreline Soft announces ₩31 bil. capital increase for operational funds
- Coreline Soft doubles AI teleradiology contract price in European deal
- Coreline Soft lands FDA clearance for upgraded AI coronary calcium analysis software
- Coreline Soft gains GDPR and HIPAA certifications for global expansion
- Coreline Soft inks China MOU with Suhai to expand AI imaging presence in Asia
- Coreline Soft strengthens Japan expansion through AI imaging deal with Micron
- Coreline Soft plugs into Bayer's AI platform to expand global lung cancer screening
- Coreline Soft expands global lung AI footprint with French project, Boehringer Ingelheim Taiwan partnership

