Medtronic said Thursday its IN.PACT AV drug-coated balloon catheter (DCB) will be reimbursed in Korea starting in May for use in dialysis patients who experience early re-narrowing of their vascular access site.
The device is used to treat stenotic lesions -- narrowed blood vessels -- in patients with end-stage renal disease (ESRD), most of whom undergo hemodialysis, a procedure that filters waste from the blood when the kidneys no longer function. In Korea, 84 percent of the country’s 18,598 ESRD patients rely on hemodialysis, typically three times a week, according to Thursday’s release.
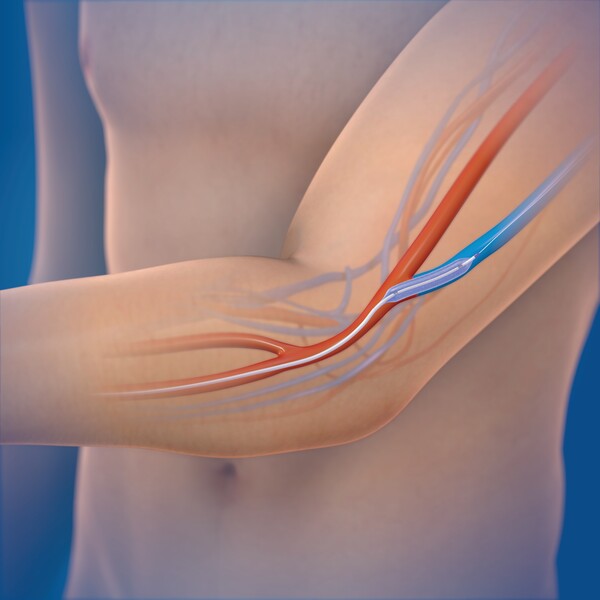
Dialysis requires a reliable access point, often created through an arteriovenous fistula (AVF) -- a surgical connection between an artery and a vein. But over time, this access can narrow or become blocked, a condition known as restenosis, often requiring balloon angioplasty, a procedure that uses a small balloon to reopen the vessel. Many patients, particularly older ones, need repeat vascular reinterventions every few months, adding physical and financial strain, Medtronic Korea said.
The IN.PACT AV DCB is coated with paclitaxel, a drug that inhibits excessive cell proliferation -- the primary cause of restenosis -- and urea, which helps the drug absorb quickly into the vessel wall. Medtronic Korea says the drug remains effective for up to 180 days. Under the new reimbursement policy, Korean patients with AVF restenosis occurring within three months of a previous angioplasty will now be eligible for the DCB treatment.
In the IN.PACT AV DCB investigational device exemption trial, Medtronic said the device reduced the need for repeat procedures by over 56 percent at six months, showing significantly longer vessel openness (primary patency) than standard angioplasty alone. At three years, patients treated with the DCB had a median target lesion patency -- how long the treated area stayed open -- of 25.4 months versus 10.7 months with conventional treatment.
“As Korea’s dialysis population continues to age, there’s been a strong need for more durable, low-burden treatment options,” said Goo Dong-erk, advisor of the Korean Society for Dialysis Access, in a statement. “Early use of IN.PACT DCB may reduce the need for repeat interventions and improve long-term outcomes.”
Yoo Seung-rok, vice president and managing director of Medtronic Korea, added, “With insurance now covering IN.PACT DCB, we’re committed to accelerating its adoption across Korean dialysis centers.”
IN.PACT AV DCB received FDA approval in 2019 for treating AVFs in ESRD patients. In Korea, the device has been used since 2012 to treat peripheral artery disease and is now expanding into dialysis access.
Related articles
- Post-Baxter, Vantive targets Korea’s kidney crisis with digital home strategy, eyes liver and lung care next
- Sudden drop in urine output? It could be acute kidney failure
- Pulsed field ablation gains momentum in Korea as game-changing atrial fibrillation treatment
- Medtronic opens Korea's 1st robotic surgery research and training center in Osong
- Medtronic to support insulin pumps for type 1 diabetes patients
- Medtronic Korea to directly sell diabetes management devices
- ‘Early detection of ANCA-associated vasculitis can stop dialysis and prevent lifelong treatment’
- SNUH 1st in Korea to adopt Medtronic’s Hugo robotic surgery system
- High-volume hemodiafiltration emerges as new standard for dialysis
- Medtronic’s leadless pacemaker hits 300,000 implants at 10 years

