Lunit, a Korean AI-powered solutions provider for cancer diagnostics, said Wednesday its chest X-ray analysis software, Lunit INSIGHT CXR4, has been granted Europe's CE certification under the more stringent Medical Device Regulation (MDR).
INSIGHT CXR4 is designed to support radiologists in a variety of clinical scenarios by detecting 12 types of chest abnormalities, including lung nodules, pneumonia, and pneumothorax, as well as additional findings such as acute bone fractures. The upgraded solution, trained using extensive real-world datasets, aims to improve diagnostic accuracy and facilitate the early detection of critical diseases.
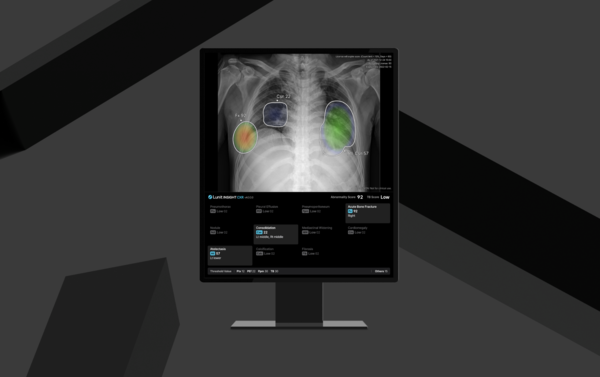
The latest software's new capabilities include an advanced "Normal Flagging" feature that utilizes AI trained on over 1.2 million chest X-rays to proactively confirm normal cases with a 99.5 percent negative predictive value, automatically generating structured reports for these low-risk exams to potentially reduce reading time. It also offers "Current-Prior Comparison" that analyzes a patient's current and previous chest X-rays, now with nodule comparison support, to track disease progression or improvement for better clinical decisions. Furthermore, "Acute Bone Fracture Detection" identifies recent traumatic fractures within the chest X-ray, aiming to reduce interpretation time and improve diagnostic accuracy, especially in high-volume settings.
With the CE MDR certification, Lunit plans to deploy INSIGHT CXR4 across Europe.
Brandon Suh, CEO of Lunit, said, "With Lunit INSIGHT CXR4, we’ve gone beyond expanding detection—we’ve focused on what truly helps clinicians in their day-to-day workflow. CE MDR certification is a key step toward broader adoption, and we’re committed to bringing CXR4 to more hospitals worldwide."
Lunit is to pursue additional regulatory approvals to make CXR4 available in more regions globally. The company's broader goal is to further integrate its AI-powered solutions into clinical workflows across diverse healthcare systems.
The CE MDR represents the European Union’s enhanced regulatory standard for medical devices, imposing stricter requirements for safety, performance, and clinical validation.
Related articles
- Lunit to unveil 12 AI-powered oncology studies at ASCO 2025
- Lunit receives approval for 3D breast tomosynthesis AI solution
- Lunit triples Q1 revenue on overseas cancer AI sales, but operating loss widens 61%
- Lunit signs AI cancer diagnostics supply deal with Germany’s largest radiology network
- SimonMed to roll out Lunit’s AI breast cancer detection software across 170 US centers
- Lunit to deploy AI imaging tools in Korean health screening clinics
- AI+radiologist collab cuts unnecessary recalls, boosts breast cancer detection accuracy: Lunit study
- Lunit partners with US National Cancer Institute to bring AI biomarkers into cancer trials
- Lunit joins forces with Microsoft to globalize cancer diagnostic AI via Azure
- Korean radiologists read 336 test images per month on average
- Lunit expands US footprint with Akumin partnership for AI breast cancer diagnostics
- VUNO, Lunit need policy clarity as deferral system deadline approches

