A Korea-U.S. research collaboration team has developed a next-generation lipid nanoparticle (LNP)-based drug delivery system that can precisely deliver therapeutic agents to neutrophils, the immune system’s first responders against bacterial and viral infections, in Covid-19 patients.
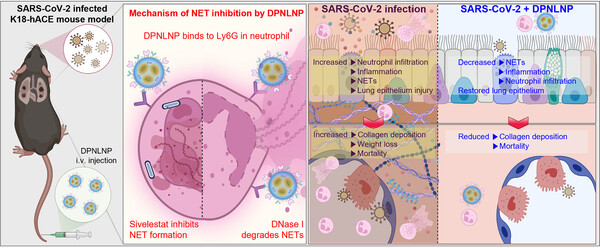
When neutrophils are overstimulated during viral illnesses such as Covid-19, they release net-like DNA-protein structures known as neutrophil extracellular traps (NETs), which not only attack pathogens but also damage healthy lung tissue.
Existing NET-inhibiting treatments have been limited in their effectiveness due to rapid degradation and poor targeting efficiency.
To address this, Professor Park Woo-ram of Sungkyunkwan University’s Department of Integrative Biotechnology, together with a research team from the University of Hawaii, developed a LNP formulation specifically designed to recognize and penetrate lung-localized neutrophils in a Covid-19 mouse model.
The system precisely delivered a NET-suppressing compound directly into neutrophils, significantly reducing the formation of NETs.
The results showed that the LNP-based therapy achieved therapeutic effects with just one-tenth the dosage of conventional approaches, demonstrating marked improvements in reducing lung inflammation and tissue damage without significant side effects.
“This is the first case to demonstrate that extracellular trap-related complications in COVID-19 and other respiratory diseases can be effectively controlled with minimal side effects by precisely targeting lung neutrophils,” Professor Park said. “The platform opens doors to future delivery of a range of immune-modulating agents into specific pulmonary cell types, and we plan to further enhance clinical feasibility through expanded global collaboration.”
The breakthrough research was supported by Korea’s Ministry of Health and Welfare and Korea Health Industry Development Institute (KHIDI) under the Global Research Collaboration Support Program from July 2023 to December 2024. The full study will be published in the Journal of Controlled Release on June 10.
Related articles
- KHIDI leads Korea’s biotech push at BIO USA with investor forum and Boston pitch event
- KHIDI and AstraZeneca support Korean biopharma’ expansion into China
- KHIDI expert calls for drug supply chain reform in Korea
- Flu pandemic advisory lifted, but Covid-19 summer outbreak remains a concern
- Korea posts all-time high in 1H health industry exports on biopharma, K-beauty strength

