Chunlab was founded based on microbiome genome analysis technology. CJ CheilJedang later acquired the company, changed its name to CJ Bioscience, and accelerated its drug development. With the financial strength of its parent company, the company is expanding its disease areas by acquiring microbiome drug pipelines from foreign companies and conducting its global clinical trials.
Although the development of microbiome drugs has slowed down at home and abroad, CJ Bioscience's business direction is consistent: to find strategies that can increase the response of immuno-oncology drugs to microbiome drugs. The company is putting all its efforts into developing this pipeline.
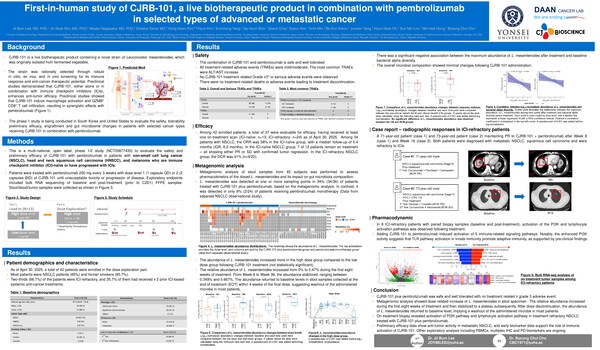
Unveils CJRB-101 phase 1/2 interim analysis, demonstrating safety and partial efficacy
CJ Bioscience presented clinical results on the safety and partial efficacy of CJRB-101 in a poster presentation at the American Society of Clinical Oncology (ASCO) this year. CJRB-101 is in early clinical trials as a strategy to increase the responsiveness of immune checkpoint inhibitors (such as Keytruda and Opdivo) in line with the current trend of anticancer drug development.
According to the company, CJRB-101 is a novel strain found in fermented foods, so its safety is assured. In a poster presented at ASCO 2025, the company reported no grade 3 or higher adverse events, serious adverse events (SAEs), or treatment discontinuations.
Microbiome drugs have the disadvantage of being difficult to produce because they are larger units than cells, which is why Korean microbiome drug development companies often have difficulty in contract manufacturing organizations (CDMOs) for clinical trials.
To overcome these limitations, CJ Bioscience identified an oxygen-resistant strain and selected CJRB-101 as the final substance. This characteristic makes it easier to mass-produce the drug.
In the end, the hurdle for microbiome drugs is apparent efficacy. Due to the strain's nature, identifying a precise mechanism of action (MOA) is difficult. It must be differentiated from lactobacillus, widely used as a healthy functional food, to show its potential as a medicine.
CJ Bioscience also announced the results of a study that clarified the MOA from the preclinical stage to overcome this hurdle in microbiome drug development and also unveiled some validation results in early clinical trials.
A poster presented at ASCO showed that CJRB-101 increases the expression of genes related to T-cell function and activation (GZMB and PRF1) and prevents the expression of immunosuppressive genes (CTLA4 and IDO1). The theory is that this MOA creates an environment that enhances the response to immune checkpoint inhibitors, including Keytruda.
In addition, a disease control rate (DCR) of 41 percent was reported in 22 patients with ICI-refractory NSCLC. The interim analysis concluded that the CJRB-101 combination may have contributed to some disease stabilization.
Non-cancer pipeline is preclinical, targeted at IBD-based follow-on clinical entry
CJ Bioscience focuses on the clinical development of its anticancer pipeline and strengthens its human resources for technology transfer. Last December, CJ Bioscience's parent company, CJ CheilJedang, hired Kim Chang-sook to head the Human BIO business. Kim has commercialization experience at Toolgen, Samsung Biologics, LG Chem, Hanmi Pharmaceutical, Novo Nordisk, and Novartis.

Recently, there has been a consensus that global cancer technology transfer is usually possible only after securing at least phase I data. In line with this situation, CJ Bioscience is also expected to pursue technology transfer by completing phase 1/2 or interim analysis results. In line with this, it is understood that CJ Bioscience has strengthened its business development personnel in earnest.
The following pipeline is for inflammatory bowel disease (IBD), the most active area for microbiome drugs. CJRB-201 is currently in the final stages of non-clinical development.
The company has obtained efficacy data for IBD in animal models and has begun producing samples for clinical entry. It is also accelerating its research, with a poster presentation of preclinical data at the European Crohn's and Colitis Congress (ECCO) in February.
However, it is a limitation that, other than the anticancer pipeline entering the clinic, the other pipelines are still in the preclinical stage. This is because it may be challenging to build a sustainable business model if CJRB-101 and others are transferred in the future.
CJRB-201 and other pipelines targeting IBD are still in the discovery and preclinical stages. In addition, pipelines for Parkinson's and asthma remain in the preclinical stage.
"We are developing differentiated new drugs based on our accumulated expertise and competitiveness in the field of microbiome drug development," a CJ Bioscience official said. "We are striving to maximize clinical efficiency and increase the chances of success in new drug development by utilizing our AI-based drug development platform ‘Ez-Mx.’”

