Leclaza (lazertinib), a lung cancer targeted therapy developed in Korea, or Tagrisso (osimertinib), the global standard?
A study that may help answer the question of which of these two third-generation EGFR tyrosine kinase inhibitors (TKIs) to choose for the initial treatment of EGFR-mutant non-small cell lung cancer (NSCLC) was published in the July issue of the internationally recognized Journal of Thoracic Oncology (JTO).
The paper presents an exploratory analysis from Leclaza's global phase 3 MARIPOSA study, which was presented by Professor Lee Se-hoon of the Department of Hematology-Oncology at Samsung Medical Center at the European Lung Cancer Society Annual Congress (ELCC 2025) in late March.
The analysis is clinically significant, as it provides a new option, Leclaza, when Tagrisso is the global standard of care for the first-line treatment of EGFR (epidermal growth factor receptor)-mutant NSCLC.
Notably, the potential of Leclaza was highlighted by the need to consider safer agents for patients who are elderly or have cardiovascular comorbidities.
First ‘randomized, double-blind’ comparison between third-generation EGFR TKIs
The MARIPOSA study was a multinational phase 3 trial that enrolled 1,074 patients. Patients were randomized in a 2:2:1 ratio to amivantamab (Rybrevant, trademark name) plus Leclaza (429 patients), Tagrisso alone (429 patients), or Leclaza alone (216 patients). This analysis only includes data comparing the two third-generation EGFR TKI monotherapies.
The study was conducted in first-line NSCLC patients with EGFR Exon 19 deletion (Ex19del) or L858R mutation, 40 percent of whom had a history of brain metastases.
Their efficacy was ‘similar’ with similar PFS, ORR, and DoR data
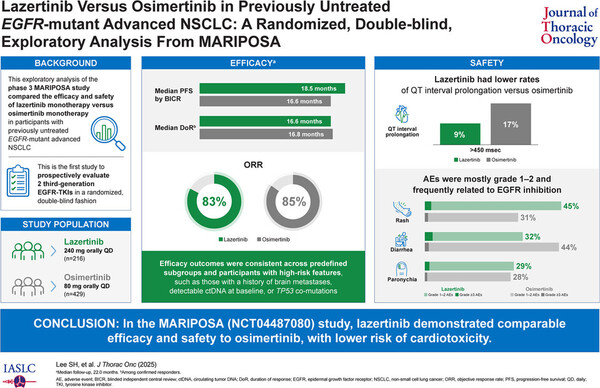
Overall, efficacy metrics did not show significant differences between the two drugs.
At 22 months of follow-up, median progression-free survival (mPFS) was 18.5 months vs. 16.6 months, objective response rate (ORR) was 83 percent vs. 85 percent, and median duration of response (mDoR) was 16.6 months vs. 16.8 months in the Leclaza and Tagrisso arms.
These results were similar in high-risk patients, including those with a history of brain metastases, TP53 mutation, and positive ctDNA.
Tagrisso reveals ‘cardiotoxicity’ concerns, as shown by longer QT and lower LVEF
The most striking difference in this analysis was in the “cardiotoxicity” metric.
QT prolongation and LVEF (left ventricular ejection fraction) decline were both lower in the Leclaza arm, which the authors attributed to Leclaza's lower HER2 inhibitory activity.
In real-world data, Tagrisso-related cardiac adverse events were reported to occur in about 5 percent of patients. They were more common in older patients with a history of atrial fibrillation or heart failure, according to the paper. Notably, there was a significant increase in mortality in patients who developed cardiotoxicity with Tagrisso treatment.
Of course, there are some adverse events where Tagrisso is favored. In particular, skin toxicity, liver enzyme elevations, and peripheral nervous system adverse events were less common in the Tagrisso arm, suggesting that Tagrisso may be more appropriate for patients with poor liver function or skin sensitivity.
Leclaza could better suit older patients with cardiovascular comorbidities
The study strongly suggests that while the overall therapeutic effectiveness of the two drugs is similar, patient comorbidities and side effect profiles may dictate which drug to choose.
The practical clinical implications are that in older patients with comorbidities such as hypertension, heart failure, and arrhythmias, Leclaza may be a safer choice due to its relatively low risk of QT interval prolongation and LVEF depression.
Previously, Tagrisso was the de facto third-generation option for the first-line treatment of EGFR-mutant lung cancer. However, this exploratory MARIPOSA analysis suggests that it is now a matter of choice, rather than effectiveness, depending on the patient's status.
In particular, in patients at risk for cardiovascular disease, older adults, and in situations where combination therapy is being considered, the advantage of Leclaza may be emphasized. At the same time, Tagrisso may still be appropriate for patients sensitive to skin and liver toxicity.
In this regard, experts regard this study as an essential milestone in the evolution of first-line treatment of EGFR-mutant NSCLC, as it demonstrates that there is no longer one absolute answer, but rather a 'personalized strategy' based on patient characteristics.
Related articles
- J&J and Yuhan push challenger to AZ’s Tagrisso in Korea with next-phase Rybrevant combo
- Yuhan secures US patent for lung cancer drug lazertinib, blocking copycats
- Yuhan collects $15 mil. as J&J lung cancer combo hits Japan
- J&J launches Yuhan-partnered lung cancer combo in Japan as AstraZeneca secures Tagrisso expansion
- J&J highlights Rybrevant-Leclaza combo’s survival benefit in EGFR-mutant lung cancer
- J&J’s Rybrevant-Lazcluze combo with Yuhan wins China approval, setting off $45 mil. milestone
- Family of lung cancer patient pleads for coverage of new drug Rybrevant
- Yuhan R&D chief steps down, leaving gap after Leclaza success

