As the body sleeps, the brain gets to work.
In a study published last month in the Journal of Cerebral Blood Flow and Metabolism, researchers in Korea say they’ve captured the brain’s self-cleaning system operating in real time during natural sleep -- without relying on contrast dyes or bulky MRI machines.
They believe it’s the first noninvasive evidence of the glymphatic system in action in living humans.
“I thought, what if we could monitor brain water the same way we track oxygen in the ICU or with a wearable device?” said Professor Yun Chang-ho, a neurologist at Seoul National University Bundang Hospital and the study’s corresponding author.
The glymphatic system, first described in a 2012 mouse study, acts like a nighttime rinse cycle. Cerebrospinal fluid flows alongside arteries, slips between brain cells, and flushes out waste, including amyloid-beta, the protein often linked to Alzheimer’s disease. But in humans, capturing this system in action has remained elusive, in part because the standard tools are either too invasive or restricted.
“You can’t ask someone to lie in an MRI and fall asleep for hours,” Yun said. The machines are loud, the space is confined, and the setting, he added, is anything but natural.
Some researchers have tried using contrast dyes to track fluid flow, but those substances aren’t approved for injection into the spinal fluid. “Only one group in Norway has permission to do that,” he said. “For everyone else, it’s off limits.”
That problem, he said, became a fixation. One night in the ICU, watching a patient’s blood oxygen levels on a monitor, Yun wondered: What if we could track brain water the same way?
That idea led to a collaboration with KAIST electrical engineer Bae Hyoun-Min and the creation of a lightweight headband that uses near-infrared spectroscopy, or NIRS, to detect minute shifts in water content.
Though NIRS is typically used to measure oxygenation in blood, the team retuned the system to focus on water. Yun, who volunteered as the device’s first test subject, said the moment he saw his own brain water levels rise and fall on the screen felt electric.
“I still remember the thrill,” he said. “That moment was unforgettable.”
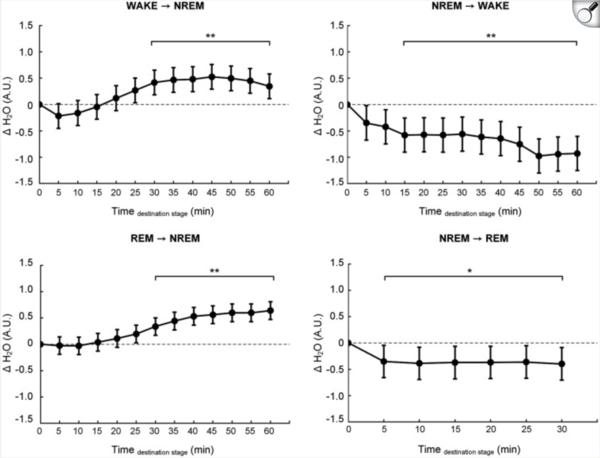
The study tracked 41 adults as they slept overnight, with water content analyzed every five minutes. Water levels rose an average of 0.57 arbitrary units as participants entered non-REM sleep. As they woke, levels dropped by 0.93 units.
Transitions between REM and non-REM also registered. The most pronounced changes, Yun said, appeared early in the night -- matching animal studies that suggest glymphatic activity peaks during the first deep sleep cycle.
The brain, Yun explained, clears itself by shrinking cells slightly during sleep, which creates space for fluid to flow in and out, removing metabolic waste. “It’s like squeezing a sponge and letting fresh fluid rinse through,” he said.
That flushing process may be more than a housekeeping trick. Disruptions to glymphatic flow have been linked to Alzheimer’s, Parkinson’s, and traumatic brain injury. But until now, studying the system in healthy people was practically off-limits.
Yun hopes the new NIRS-based approach could serve as a stepping stone toward wearable diagnostics. These tools could one day monitor brain waste clearance as a proxy for cognitive health, he said. Though still experimental, the headband could become a biomarker for sleep quality or neurodegenerative risk.
“We’ve had a bottleneck,” he said. “We haven’t had a way to study this in healthy people, during real sleep, without invasive tools. That’s starting to change.”
The implications, he added, may extend far beyond the lab. “If we can measure how well your brain is cleaning itself each night,” he said, “maybe we can change how we think about aging altogether.”

