The treatment environment for pulmonary arterial hypertension (PAH), which has been extremely limited in Korea, is seeing new possibilities with the recent introduction of new drugs.
The Ministry of Food and Drug Safety (MFDS) approved Opsynvi, a combination therapy for PAH, for which Janssen Korea applied, last Thursday.
Opsynvi combines macitentan, an endothelin receptor antagonist (ERA), and tadalafil, a phosphodiesterase type 5 (PDE-5) inhibitor, into a single tablet, thereby enhancing patient convenience and potentially marking a significant turning point for combination therapy in patients with PAH.
Additionally, a day before Opsynvi, MSD Korea's Winrevair (sotatercept) received approval from the MFDS, further expanding treatment options for patients with PAH.
Winrevair is the first-in-class Activin Signaling Inhibitor (ASI) approved in the PAH field, marking the first new mechanism of action in 20 years.
This drug blocks excessive signaling of activin, a protein that promotes cell proliferation in pulmonary arteries, restoring balance with anti-proliferative signals. That induces reverse remodeling, normalizing altered vascular structures and directly targeting the underlying cause of the disease.
Winrevair has been recognized for its innovation and was designated as the 24th drug under the MFDS’ Global Innovative Products on Fast Track (GIFT) program in 2024. It has also been selected as a target for the second parallel approval, evaluation, and negotiation pilot program. It is expected to be submitted to the Pharmaceutical Reimbursement Evaluation Committee (PREC) for review soon.
Additionally, in June, Bayer Korea's Adempas (riociguat) was included in the national health insurance reimbursement list 11 years after receiving approval from the MFDS. Adempas is a soluble guanylate cyclase (sGC) stimulator that acts independently of the nitric oxide (NO) pathway, providing an additional treatment option for patients who have not responded adequately to existing ERA and PDE-5 inhibitors, and expanding the possibilities for combination therapy.
PAH is a rare and severe intractable disease characterized by abnormal thickening and narrowing of the walls of the pulmonary arteries, leading to increased pressure. According to a recent report by the Korean Society of Pulmonary Hypertension, the number of patients with PAH in Korea was estimated to be approximately 6,000 in 2023. However, the five-year survival rate for PAH patients in Korea remains at around 70 percent, with an average survival period of only 13.1 years.
This is significantly lower than the five-year survival rate (85 percent or higher) in other developed countries, including Japan. The difficult diagnosis of PAH in the Korean medical environment and low treatment accessibility are cited as the main causes.
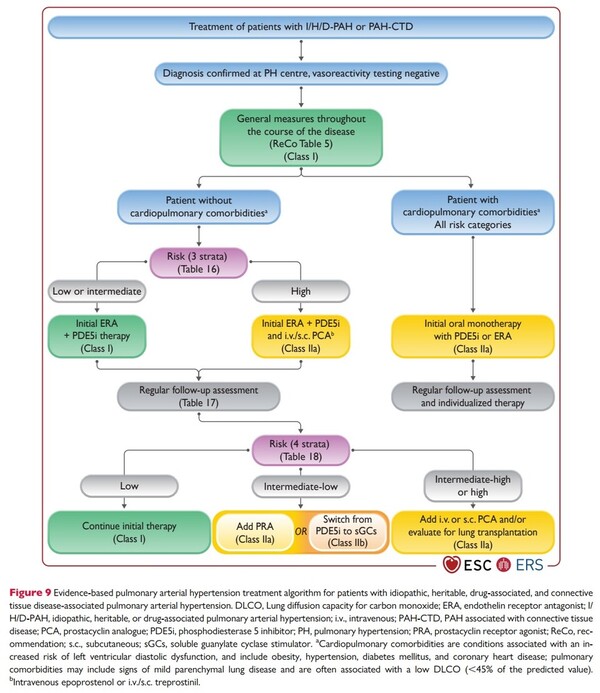
Aside from the Korean situation, the paradigm of PAH treatment is rapidly evolving. According to the ESC/ERS Pulmonary Hypertension Guidelines published by the European Society of Cardiology (ESC) in 2022, combination therapy with ERA and PDE5i is recommended from the outset. For high-risk patients, combination therapy centered on intravenous or subcutaneous injection of prostacyclin agents (PCA), such as epoprostenol, is mandatory. The strategy of simultaneously blocking multiple treatment pathways from the initial diagnosis, rather than gradually increasing a single agent, is becoming the global standard.
Opsynvi, Adempas, and Winrevair target different pathways—ERA, PDE-5, sGC stimulators, and activin inhibitors—and can contribute to the implementation of the initial combination therapy strategy outlined in the guidelines. In particular, Winrevair is a new class of drugs that can “fundamentally modify” the disease and is likely to be included in future treatment guidelines.
Therefore, the approval and reimbursement of these drugs are expected to be a turning point that will dramatically raise the level of PAH treatment in Korea, going beyond simply expanding treatment options. In particular, with the realization of early combination therapy, it is expected to play a decisive role in increasing the survival rate of patients and transforming the disease into a “manageable disease.”
Core treatments for high-risk patients remain ‘blank’
Nevertheless, prostacyclin injections, which are core treatments for high-risk PAH patients, have not yet been introduced in Korea, leaving the treatment system incomplete. This means that there is still a critical treatment gap for patients whose lives are at risk.
Epoprostenol, developed 30 years ago, remains the only drug not introduced in Korea among OECD member countries. GSK, which holds the original drug Prolan, has no plans to launch it in this market. Global guidelines recommend this drug as the “first-line treatment option” for high-risk patients, but it remains unavailable in Korea.
Recently, various new drugs with different mechanisms, such as Opsynvi, Adempas, and Winrevair, have been successively approved and incorporated into the reimbursement system in Korea. This has gradually diversified the Korean treatment strategy, which was previously limited to a single pathway. As a result, the foundation has been laid for practically implementing the combination therapy strategy recommended by global guidelines in Korea, thereby increasing the likelihood of patient-tailored treatment in actual clinical practice.
The remaining challenge is to fill the gaps in essential medications for high-risk patients and revise Korea’s treatment guidelines to reflect these changes. It remains to be seen how the Korean PAH treatment guidelines, which have been stagnant, will be revised in line with the recent shift toward new drug-centered treatment paradigms, and whether Korean patients can fully benefit from treatment that aligns with global standards.
Related articles
- MSD scores Korean approval for pulmonary arterial hypertension drug Winrevair
- Bayer's Adempas gets insurance coverage in Korea 11 years after approval
- PNH care faces a turnaround, but the new drug’s reimbursement remains an issue
- Experts urge government support for life-saving pulmonary arterial hypertension drugs
- MSD’s Winrevair arrives in Korea but meets hurdles of older PAH drugs
- Physicians decry 30-year gap in PAH treatment access at policy debate
- Korean PAH patients deperately hope GSK ends 30 years of inaction
- 'High blood pressure is never healthy -- even in old age'
- 'Sotatercept slashes death risk by 76% in pulmonary arterial hypertension'

