Deepnoid has cleared a major hurdle in Korea, winning regulatory approval to begin clinical testing of its generative AI radiology device M4CXR.
The Ministry of Food and Drug Safety signed off Tuesday on the company’s trial plan, opening the door to what Deepnoid calls the first large-scale test of a radiology tool built on a large language model rather than a traditional neural network.
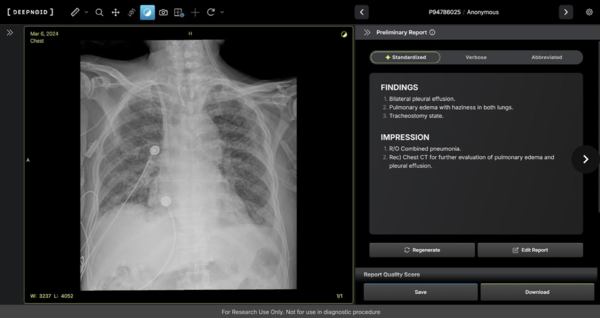
The company will run the study with Kangbuk Samsung Hospital and Boramae Medical Center, aiming to prove the device’s accuracy and safety.
Deepnoid said M4CXR can scan chest X-rays and draft a preliminary report in seconds, flagging 41 types of lesions based on training from more than 10 million X-rays and corresponding reports. The company added that such speed could help doctors in emergencies where time is critical.
“Chest X-rays are the most frequently performed imaging exam, but the shortage of radiologists has limited timely interpretation,” said Kim Sung-hyun, chief director of the Human Medical Imaging and Intervention Center. “This system can deliver preliminary results instantly, improving diagnostic speed in emergencies and helping radiologists achieve greater accuracy.”
Deepnoid said it plans to launch the trial next month, then move toward product approval and market entry through Korea’s fast-track pathway for non-reimbursed use before seeking insurance coverage.
Related articles
- Can generative AI transform medical imaging and diagnosis?
- [Interview] Deepnoid bets on Japan as AI testing ground before a push into US
- Deepnoid adopts DeepSeek R1 while Korean government bans the model over security risks
- ‘Deepnoid’s M4CXR outperforms ChatGPT in chest X-ray’
- Deepnoid and GeneCast to codevelop early cancer-detection tech

