Brightonix Imaging announced Tuesday that its clinical positron emission tomography (PET) system, PHAROS, has received the U.S. Food and Drug Administration’s 510(k) clearance on Aug. 15, marking the first FDA approval for a homegrown PET scanner in Korea.
The company said the clearance validates the safety and performance of the system on an international scale, paving the way for a new era in precision nuclear medicine imaging and positioning Brightonix Imaging as a leading innovator in medical technology.
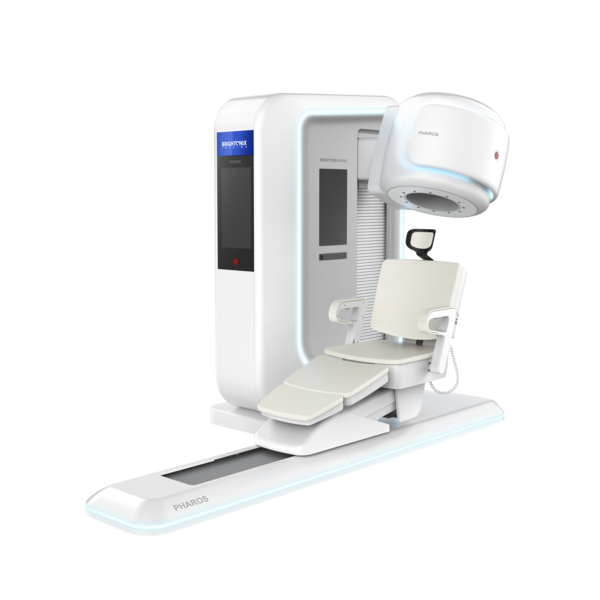
PHAROS integrates advanced depth-of-interaction (DOI) and time-of-flight (TOF) technologies to deliver high resolution and sensitivity, enabling accurate and reproducible PET imaging results across diverse clinical settings. The system is designed to support more precise diagnoses and efficient clinical decision-making for physicians.
Unlike conventional PET scanners, PHAROS is a multifunctional system that allows adjustments of the patient seat and detector positions for different body regions, including the brain, breast, and extremities.
For brain imaging, the system supports both supine and seated positions, enhancing patient comfort while improving workflow efficiency.
“The FDA clearance of the PHAROS PET scanner is highly significant not only for Brightonix Imaging but also for the entire medical imaging field,” Brightonix Imaging CEO Lee Jae-sung said. “This technology will enable clinicians to diagnose and treat neurodegenerative diseases earlier and more accurately, ultimately improving patient outcomes.”
With FDA clearance now secured, Brightonix Imaging plans to strengthen its presence in the global nuclear medicine market and advance as a leader in next-generation digital medical imaging solutions.

