A combination of amivantamab and lazertinib therapy in the first-line treatment improved overall survival compared with the existing targeted therapy, osimertinib, in EGFR-mutated advanced lung cancer, a phase 3 trial showed.

A research team led by Professor Cho Byoung-chul of Yonsei Cancer Center’s Lung Cancer Center reported on Tuesday that in a multinational, randomized phase 3 trial involving untreated patients with EGFR-mutated, locally advanced or metastatic non-small cell lung cancer (NSCLC), the combination of amivantamab and lazertinib improved the three-year survival rate by 9 percentage points compared with the current standard therapy, osimertinib.
The findings were published in the New England Journal of Medicine (impact factor 176). This marks the first time a Korean oncologist, Professor Cho Byoung-chul, has had three studies published in the prestigious journal, and also the first time clinical results of a homegrown cancer drug have appeared in NEJM twice.
EGFR-mutated NSCLC accounts for 25 to 40 percent of all lung cancer cases and is the most common subtype, with an estimated 450,000 new patients diagnosed globally each year. Until now, the standard first-line therapy, osimertinib, has shown a response rate of about 80 percent and a progression-free survival of 16 to 18 months. However, most patients eventually develop resistance.
After phase 1 and 2 trials demonstrated the combination therapy’s efficacy, Professor Cho proceeded with a multinational, randomized phase 3 study. The regimen included amivantamab, approved by the Ministry of Food and Drug Safety for advanced EGFR-mutated NSCLC, and lazertinib, approved also by the MFDS for NSCLC driven by specific EGFR exon 20 mutations. The combination therapy had previously demonstrated superior progression-free survival over osimertinib, leading to U.S. FDA approval in August 2024.
Among 429 patients treated with the combination regimen, the three-year overall survival rate was 60 percent, an improvement of 9 percentage points over the osimertinib arm.
During the 37.8-month follow-up, the median overall survival of the amivantamab–lazertinib group “surpassed the observation period,” whereas the osimertinib group recorded 36.7 months, Severance Hospital said.
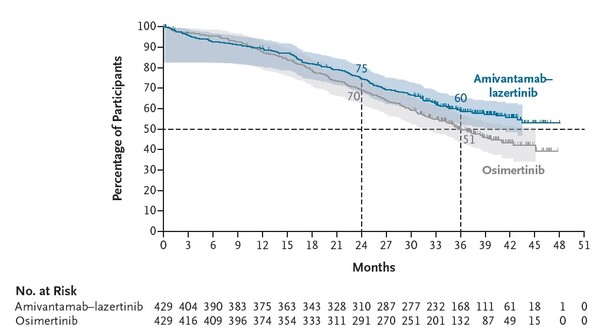
The combination therapy also demonstrated superior efficacy in patients with brain metastases. Its main side effects were skin rash and paronychia, but most were mild reactions that could be managed.
“By demonstrating not only improved progression-free survival last year but now also improved overall survival, we have confirmed the clear therapeutic benefit of the amivantamab-lazertinib combination therapy for patients with advanced EGFR-mutated NSCLC,” Professor Cho said.
“With this NEJM publication, we present the possibility that a first-line chemotherapy-free strategy using next-generation targeted therapies could become a new standard of care, surpassing conventional chemotherapy.”
Related articles
- ACROBiosystems and Daan Biotherapeutics collaborate on developing immuno-oncology drugs
- Yonsei Cancer Center study identifies MET gene as key target across solid tumors
- Yonsei Cancer Center presents positive P1 results for next-gen KRAS G12C inhibitor treatment
- Will Leclaza-Rybrevant combo therapy open new horizon for EGFR lung cancer treatment?
- NEJM publication of amivantamab+lazertinib combo increases anticipation of FDA approval
- Yonsei Cancer Center to host OncoRun marathon for survivors in November
- Yonsei Cancer Center spotlights patient-reported outcomes to enhance cancer care
- ‘In EGFR-mutated lung cancer treatment, combo therapy emerges as new standard’

