Ybrain, a Korean mental health-focused digital therapeutics platform, said on Wednesday that its electroencephalogram (EEG) analysis device, MINDD SCAN, has surpassed 300,000 cumulative prescriptions.
Approved by the Ministry of Food and Drug Safety in 2019, MINDD SCAN is prescribed under national health insurance coverage in neurology and psychiatry departments in Korea.
In particular, MINDD SCAN simultaneously measures EEG and heart rate variability (HRV), enabling the detection of brain functional abnormalities that cannot be identified by conventional CT or MRI scans.
CT and MRI are imaging tests that show the brain's shape and structure, and are effective at detecting physical damage like brain tumors, hemorrhages, or cerebral infarction.
However, functional abnormalities such as depression, anxiety, ADHD, sleep disorders, or chronic pain do not show up structurally, making them difficult to identify with CT or MRI alone.
In such cases, brainwave analysis tools like MINDD SCAN are valuable, as they analyze the brain's electrical activity patterns to visualize and evaluate functional issues.
According to Ybrain, MINDD SCAN is used in over 310 psychiatric and neurological hospitals and clinics in Korea. Since the start of this year, it has averaged over 10,000 measurements per month, with cumulative measurements exceeding 304,000 to date.
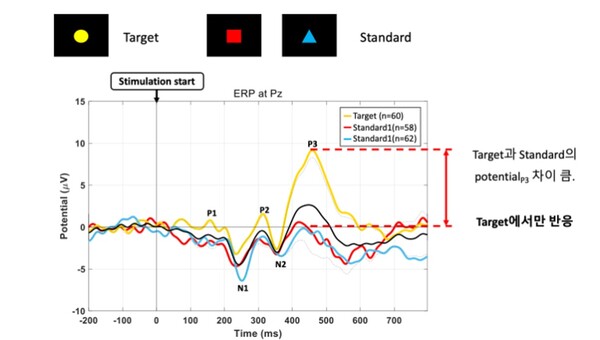
During a brainwave measurement simulation using MINDD SCAN at the Korean Society of Neurophysiology's Brainwave School in August, MINDD SCAN displayed P300 graphs, proving the accuracy of its brainwave measurements. P300 is an indicator reflecting the speed at which the brain recognizes and processes stimuli. It evaluates brain attention, cognitive ability, and brain health status based on the time it takes the brain to recognize surprising or noteworthy stimuli, using 0.3 seconds as a benchmark.
“MINDD SCAN digitizes and statistically analyzes brainwaves, objectively presenting patterns of over-excitation, under-activity, and abnormal connectivity compared to normal groups, aiding in the scientific diagnosis of patients' conditions,” said Dr. Cho Geun-ho, Director of Cho Geun-ho Psychiatric Clinic. “The demand for data-based diagnosis among patients is expected to continue growing.”
Lee Ki-won, CEO of Ybrain, said the company expects MINDD SCAN's objective diagnostic support system to be of great help in patients' treatment.
Related articles
- Ybrain ships depression therapy device to Thailand
- Ybrain's home-use depression device granted extended access in Korea
- Ybrain’s drug-free depression device tops 180K prescriptions in Korea
- Ybrain launches digital sleep diary for clinical diagnosis of insomnia
- Ybrain proposes context-based adaptive interfacing at International BCI Meeting in Korea
- Ybrain's device improves depression symptoms in perinatal women: data
- Ybrain selected for government-funded project to develop wearable robots for paralysis rehabilitation
- Increase in insulin resistance sharply raises risk of depression
- Electronic antidepressant MINDD STIM shows strong early results in perinatal depression trials
- Ybrain launches at-home depression electroceutical MINDD STIM+ Pro

