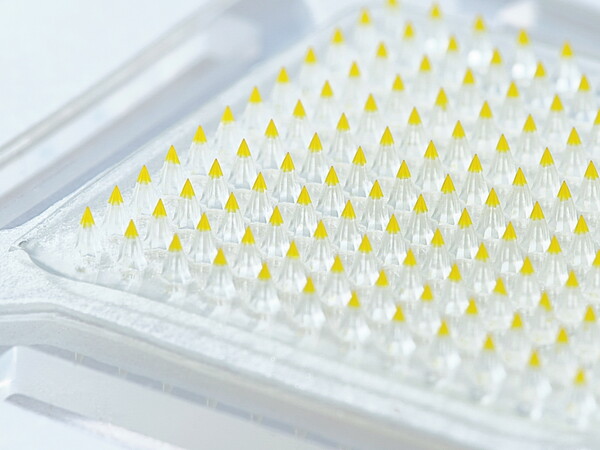
Daewoong Pharmaceutical and its affiliate Daewoong Therapeutics said Wednesday they have won approval from Korea’s Ministry of Food and Drug Safety to begin a phase 1 trial of DWRX5003, a microneedle patch that delivers semaglutide for obesity.
The first-in-human trial will assess safety and pharmacokinetics in healthy adults and measure relative bioavailability against Novo Nordisk’s injectables Ozempic and Wegovy.
The once-weekly patch uses dissolving microneedles to deposit drug into the dermis and is built on Daewoong Therapeutics’ CLOPAM platform, which employs pressurized drying and sealed packaging to stabilize dosing.
The company says CLOPAM has shown more than 80 percent bioavailability versus subcutaneous injection, well above the roughly 30 percent reported for earlier microneedle patches and about 160 times that of oral formulations.
Daewoong argues a self-applied patch could cut clinic time tied to injections and improve adherence for patients who want a needle-free option.
Related articles
- Korean and US experts say Wegovy is redefining obesity care, but access is key
- Daewoong’s semaglutide microneedle patch hits 80% absorption, challenges injection dominance
- [Reporter’s Notebook] Sam Chun Dang Pharm’s misleading hype over oral GLP-1 trial data
- Wegovy ushers in double-digit weight loss era, redefining metabolic disease treatment
- Daewoong confirms safety and continued progress of P2 trial for IPF drug
- Daewoong showcases microneedle, biosimilar tech at CPHI Worldwide 2025

