Korea's Hanmi Pharmaceutical has asked the U.S. FDA to clear a first-in-human study of LA-UCN2 (HM17321), an obesity candidate the company says can trim fat while increasing muscle mass.
The phase 1 trial in healthy adults will assess safety, tolerability, pharmacokinetics and pharmacodynamics.
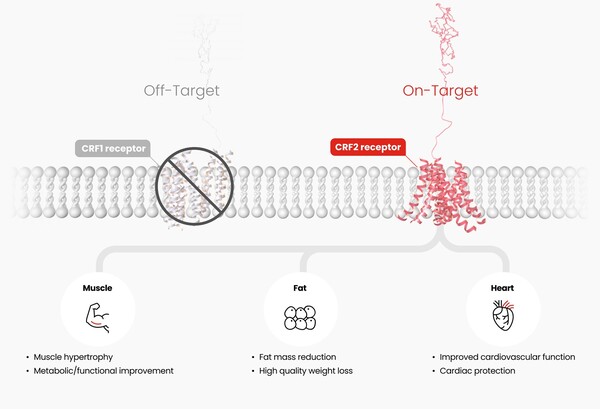
HM17321 is a peptide analog of urocortin-2 that selectively targets the corticotropin-releasing factor 2 receptor, rather than incretin receptors such as GLP-1. Hanmi says the molecule was designed with in-house AI and structural modeling and is intended to drive fat reduction, muscle growth and improved function.
The pitch rests on a string of nonclinical readouts. Since unveiling early data at ObesityWeek 2024, Hanmi has reported results in cynomolgus and rhesus monkey models that showed selective fat-weight loss with preservation of lean mass. At this year’s European Association for the Study of Diabetes (EASD 2025), the company said it also mapped a pathway consistent with resistance-training-like muscle hypertrophy. The claims remain preclinical and unproven in humans.
Hanmi is positioning HM17321 as a stand-alone therapy and a potential add-on to incretin drugs. Because it is a peptide, the company argues it could be co-administered with GLP-1–based agents and may offer dosing convenience versus intravenous antibody approaches that aim to preserve muscle.
Hanmi set a 2031 launch goal for HM17321, part of its “H.O.P” obesity franchise alongside efpeglenatide, which targets domestic approval in the second half of 2026, and HM15275, a long-acting GLP-1/GIP/glucagon triple agonist that received FDA clearance in July to begin phase 2 and is slated to start enrollment soon.
“We do not see obesity treatment as a simple race to cut weight,” R&D Center head Choi In-young said, adding that HM17321 is being developed for “integrated efficacy” that includes fat loss, muscle gain and functional improvement. Hanmi highlighted potential use in sarcopenic obesity and older patients if the effect translates clinically.
Hanmi plans four presentations on HM17321 and HM15275 at ObesityWeek 2025 in Atlanta in November.
Related articles
- Hanmi Pharm files phase 3 IND for GLP-1 combo in type 2 diabetes
- Gilead licenses Hanmi’s encequidar for global virology in $34.5 mil deal
- Hanmi Pharm signs deal with Silanes to supply diabetes combo drug to Mexico
- Hanmi’s triple-combo Amoprel sets new standard in hypertension care
- Hanmi Science and Hanmi Pharmaceutical get ‘AA-’ rating from NICE
- Hanmi Pharm continues to unveil new anticancer drug pipelines globally
- Hanmi Pharm signs contract with Viet Phap to supply hypertension combo drugs

