Pfizer’s oral Covid-19 treatment Paxlovid helped Covid-19 patients aged 65 or older reduce hospitalization and death rates, but the effect was nonexistent among younger adult patients, a study showed.
The Clalit Research Institute in Israel and Harvard Medical School in the U.S. published their study in the New England Journal of Medicine (NEJM) on Wednesday. The study analyzed the relationship between Paxlovid administration and hospitalization and death rates in 109,254 Covid-19 patients from Jan. 9 to March 31, based on patient data from Clalit Health Services.
Among the 109,254 patients, 3,902 (4 percent) took Paxlovid at least once. They include 2,484 patients aged 65 or more and 1,418 patients aged between 40 and 64.
The research team divided the patients into Paxlovid-treated and untreated groups. Then, they compared the hospitalization and death rates according to age groups and treatment status.
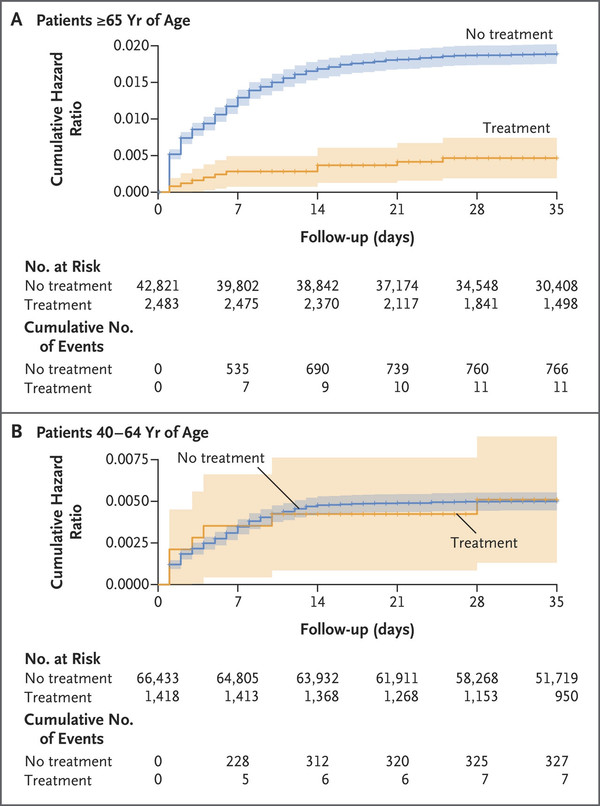
The results showed that Paxlovid was effective in patients aged 65 or more but not beneficial for those aged 40-64.
Eleven were hospitalized among Covid-19 patients aged 65 or older who took Paxlovid, 11 were hospitalized. This was equivalent to 14.7 per 100,000 people.
In contrast, 766 were hospitalized among the untreated, equivalent to 58.9 per 100,000.
Paxlovid’s therapeutic effect was little in patients aged 40-64. Among the treated group aged 40-64, 15.2 per 100,000 were hospitalized. Among the untreated, 15.8 per 100,000 were hospitalized, showing no significant difference.
Paxlovid’s reduction of death risk was greater in the elderly group.
The hazard ratio of Paxlovid-treated patients aged 65 or more went down to 0.21, while the HR of those aged 40-64 was 1.32 even after Paxlovid treatment.
“Among patients 65 years of age or older, the rates of hospitalization and death due to Covid-19 were significantly lower among those who received nirmatrelvir (Paxlovid) than those who did not. However, no evidence of benefit was found in younger adults,” the research team said.
The research team noted that, as in any retrospective cohort study, various confounders may have caused bias in the observed efficacy.
“Future studies will be needed to assess the short- and long-term safety of nirmatrelvir treatment in real-world settings,” it added.

