VUNO said on Tuesday that its artificial intelligence (AI)-based bone age reading assistance solution, VUNO Med-BoneAge, has obtained medical device certification from the Taiwanese Food and Drug Administration (TFDA).
Vuno had already signed a local distribution contract with CHC Healthcare Group, Taiwan's largest general medical company, in early 2021.
With the latest approval, Vuno said it would start selling the product in Taiwan.
CHC signed an agreement with Vuno for four major products in the medical imaging field in Taiwan. The Taiwanese company is responsible for obtaining local licenses, marketing, promotion, sales and maintenance services, said a Vuno official.
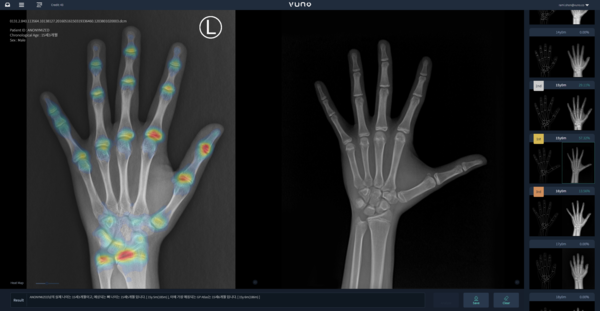
Vuno Med-BoneAge is the first AI medical device in Korea which helps to read bone age by analyzing X-rays of the hand bones based on AI.
AI automatically analyzes the bone X-ray image and presents the three most similar bone ages to assist doctors in reading bone age. Also, reports containing useful growth information for patients, such as predictive growth keys, are also generated to increase the satisfaction of physicians and patients.
Taiwan has a global level ICT infrastructure and is rapidly emerging as a powerhouse in digital healthcare in Asia.
Vuno's local partner, CHC, is a company with abundant medical device sales expertise and know-how accumulated over 40 years in Taiwan, large hospitals affiliations, and various local hospital networks.
"Vuno Med-BoneAge is currently active in more than 400 medical institutions in Korea and is an excellent product that has proven clinical efficacy," said Vuno CEO Lee Ye-ha. " In addition to Vuno Med-Chest X-ray, which obtained the Taiwanese license last year, we will actively engage in business with local partners so that Vuno's products can be widely used in Taiwan's medical field."

