VUNO, a Seoul-based medical AI solution company, said Tuesday that it won approval for VUNO Med-Fundus AI, an AI-based fundus reading device, from Taiwan’s Food and Drug Administration(TFDA).
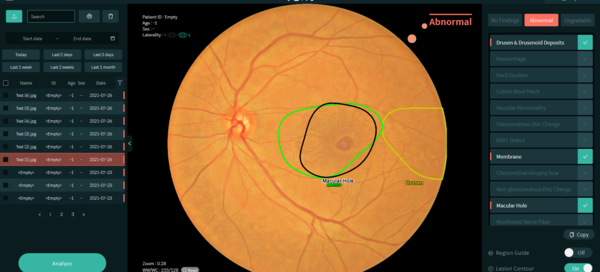
The company said VUNO Med-Fundus AI, designed for diagnosis of retinal diseases based on fundus images, was the first of its kind in Korea. More specifically, the device analyzes fundus images and informs doctors of the location of lesions essential for diagnosing retinal diseases such as diabetic retinopathy, macular denegeration, and glaucoma.
With the latest nod, VUNO has three authorized AI medical devices in Taiwan. The other two are VUNO Med-Chest X-ray and VUNO Med-BoneAge.
VUNO Med-Fundus AI was named Korea's No.1 innovative medical device by the Ministry of Food and Drug Safety in July 2020.
The company said it would cooperate with CHC Healthcare Group, a Taiwanese healthcare firm, to reinforce the sales of VUNO Med Solution using CHC Healthcare’s local networks of hospitals.
"The medical device approval set a foundation to strengthen the business outcome of VUNO Med Solution in Taiwan," VUNO CEO Lee Ye-ha said.

